Abstract
We recently described lack of phenotypic plasticity in reproductive organ development and substantial plasticity in vegetative organ development for the cycad Cycas micronesica. Is there an evo-devo explanation for the disparity in phenotypic plasticity of vegetative vs. reproductive organs? Despite modularity, might evolution of cycad phenology be controlled more by drift than selection?
Introduction
Understanding the developmental patterns of vegetative and reproductive organs and factors that influence those patterns is of critical importance for conserving rare plants. We recently determined that leaf and leaflet expansion of the endangered Cycas micronesica were influenced by spatial and temporal factors, but reproductive organ expansion was unaffected or only minimally influenced by the same factors.Citation1 We pointed out that empirical approaches for fitting models to organ growth and development may be used to inform horticultural or conservation questions of other rare cycad taxa. Here we argue that this approach may improve our understanding of the evolutionary and developmental biology of cycads.
Modularity and Endoploidy in Cycads
While slow to gain traction in botany, especially in studies of gymnosperms (but see refs. Citation2 and Citation3), evo-devo has become one of the most promising and productive perspectives in biology. This should not be surprising given that development describes processes within a generation and evolution describes processes among generations. In writing about fossil cycads, MamayCitation4 first suggested that angiosperm carpels evolved from megasporophylls, possibly of cycads. In more modern terms, examining regulatory genes, FrohlichCitation5 suggested that angiosperm carpels evolved from microsporophylls. These two hypotheses are probably incommensurate given that female and male strobili of cycads are homologous, unlike strobili of all other extant gymnosperms.Citation6 Cycad megastrobili and microstrobili are effectively a telescoped flush of leaves (). Both female and male strobili are still highly modified, so we still expect different developmental pathways in vegetative leaves, megastrobili and microstrobili. Development in cycads is undoubtedly modular, as in other gymnosperms,Citation7 although maybe not as modular as in angiosperms.Citation8 Thus ontogeny of various cycad organs should progress differently from other organs in the same individual plant.
We expect different developmental timing in vegetative vs. reproductive organs because cycad strobili are likely highly endoploid. Most male cycad strobili are thermogenic.Citation9-Citation12 Thermogenesis probably requires extra mitochondria, as we see in several skunk cabbage (Symplocarpus) species.Citation13,Citation14 While almost exclusively documented in angiosperms, plants have plenty of endoploidy, especially succulent plants,Citation15 which might include stems and strobilus axes in cycads. Furthermore, animal tissues with high metabolic demand are often highly endoploid, such as heart muscles, flight muscles and liver cells.Citation16 We suspect that endoploidy provides a way around otherwise rate-limiting production of mRNA. Efforts to confirm endoploidy in cycad strobili are warranted because endoploidy alters developmental rates insofar as mitotic cycle rates are inversely proportional to ploidy levelsCitation17 and probably inversely proportional to chromosomal content per nucleus, i.e., C-values.
Environment and Cycad Evolution
There are four modes of evolutionary response to changing environments: (1) environmental tracking, (2) phenotypic plasticity, (3) bet hedging, and (4) extinction.Citation18 A priori, we expect greater environmental tracking and phenotypic plasticity in vegetative structures because the individual plant cannot grow without leaves. However, we expect more bet hedging (risk aversion) in reproductive organ production for any perennial plant; especially in Cycas micronesica, which makes large investments in both female and male strobili.Citation19 But these are all mechanisms by which organisms evolve via selection. By contrast, Gorelick and OlsonCitation20 argued that drift should play a disproportionate role in cycad evolution compared with selection because of the peculiar genomic architecture and small population sizes (see also ref. Citation21). Similarly, Lynch and ConeryCitation22 hypothesized that large genome size, which we see in cycads,Citation20,Citation23 may be maladaptive. Thus developmental rates of different plant parts may be nothing more that what Gould and LewontinCitation24 mistakenly called spandrels. Maybe there are no adaptationist explanations nor fitness benefits to the developmental patterns we described.Citation1 Or, alternatively, maybe these developmental patterns are nothing more than a corollary of endoploidy levels, which we presume are greater in thermogenic cones than in other plant parts.
Conclusions
Cycads are a threatened group of plants worldwideCitation25 for which recovery plans have already been proposed or implemented for some taxa (e.g., see ref. Citation26). A full understanding of evolutionary developmental trajectories of cycads may be a prerequisite for restoring habitats during recovery efforts. Thus far, we have only examined phenology and evolutionary responses in the most basal genus of cycads, Cycas, with its disaggregated female strobili. Do Zamiaceae also show lack of phenotypic plasticity in cone development? Because of the putative recent ancestry of all extant cycads,Citation27 we anticipate similarities among all living cycads, which may not be due to any selective advantage. Does plasticity of cycad male cone development differ from that of other gymnosperms? Because cycad male cones are presumably not homologous with those of other extant gymnosperms,Citation6 the relative roles of selection and drift may not be the same in evolution of cycads vs. other gymnosperms. Given that cycads are the most basal of living seed plants, it is incumbent upon us to better understand these fascinating plants. Measurement of effective population sizes, plasticity, heterochrony, and endoploidy will help immensely in answering these evolutionary questions about cycads and may well help in their conservation.
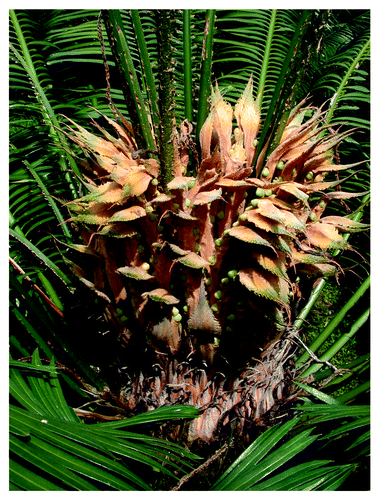
Figure 1. A Cycas seemannii megastrobilus (or ‘indeterminate strobilus’ sensu in ref. Citation28) emerges as a flush of megasporophylls bearing naked ovules, which are almost certainly homologous to the pinnately compound vegetative leaves. The genus Cycas contain some species with pinnately compound megasporophylls, e.g., C. revoluta and C. pectinata,Citation11 while all members of the genus have new flushes of vegetative leaves that emerge from the center of the megastrobilus, as seen in this figure, further indicating homology between sporophylls and vegetative leaves in cycads.
Acknowledgments
Root Gorelick thanks the Natural Sciences and Engineering Research Council of Canada (NSERC) for financial support.
References
- Marler TE, Dongol N. Models to describe Cycas micronesica leaf and strobili development. HortScience 2011; 46:1333 - 7
- Abercrombie JM, O’Meara BC, Moffatt AR, Williams JH. Developmental evolution of flowering plant pollen tube cell walls: callose synthase (CalS) gene expression patterns. EvoDevo 2011; 2:14; http://dx.doi.org/10.1186/2041-9139-2-14; PMID: 21722365
- Frohlich MW. MADS about Gnetales. Proc Natl Acad Sci U S A 1999; 96:8811 - 3; http://dx.doi.org/10.1073/pnas.96.16.8811; PMID: 10430847
- Mamay SH. Paleozoic origin of cycads. US Geol Surv Prof Pap 1976; 934:1 - 48
- Frohlich MW. An evolutionary scenario for the origin of flowers. Nat Rev Genet 2003; 4:559 - 66; http://dx.doi.org/10.1038/nrg1114; PMID: 12838347
- Mundry M, Stutzel T. Morphogenesis of male sporangiophores of Zamia amblyphyllidia D. W. Stev. Plant Biol 2003; 5:297 - 310; http://dx.doi.org/10.1055/s-2003-40791
- Vázquez-Lobo A, Carlsbecker A, Vergara-Silva F, Alvarez-Buylla ER, Piñero D, Engström P. Characterization of the expression patterns of LEAFY/FLORICAULA and NEEDLY orthologs in female and male cones of the conifer genera Picea, Podocarpus, and Taxus: implications for current evo-devo hypotheses for gymnosperms. Evol Dev 2007; 9:446 - 59; http://dx.doi.org/10.1111/j.1525-142X.2007.00182.x; PMID: 17845516
- Williams JH. Novelties of the flowering plant pollen tube underlie diversification of a key life history stage. Proc Natl Acad Sci U S A 2008; 105:11259 - 63; http://dx.doi.org/10.1073/pnas.0800036105; PMID: 18678915
- Tang W. Heat production in cycad cones. Bot Gaz 1987; 148:165 - 74; http://dx.doi.org/10.1086/337644
- Tang W. Heat and odour production in cycad cones and their role in insect pollination. In: Stevenson DW, Norstog KJ, eds. The biology, structure, and systematics of the Cycadales. Proceedings of Cycad 90, the second international conference on cycad biology. Palm & Cycad Societies of Australia, Milton, 1993:140-7.
- Norstog KJ, Nicholls TJ. The biology of cycads. Cornell University Press, Ithaca. 1997.
- Terry I, Moore CJ, Walter GH, Forster PI, Roemer RB, Donaldson JD, et al. Association of cone thermogenesis and volatiles with pollinator specificity in Macrozamia cycads. Plant Syst Evol 2004; 243:233 - 47; http://dx.doi.org/10.1007/s00606-003-0087-x
- Knutson RM. Heat production and temperature regulation in eastern skunk cabbage. Science 1974; 186:746 - 7; http://dx.doi.org/10.1126/science.186.4165.746; PMID: 4417289
- Ito-Inaba Y, Hida Y, Inaba T. What is critical for plant thermogenesis? Differences in mitochondrial activity and protein expression between thermogenic and non-thermogenic skunk cabbages. Planta 2009; 231:121 - 30; http://dx.doi.org/10.1007/s00425-009-1034-z; PMID: 19859730
- De Rocher EJ, Harkins KR, Galbraith DW, Bohnert HJ. Developmentally regulated systemic endopolyploid in succulents with small genomes. Science 1990; 250:99 - 101; http://dx.doi.org/10.1126/science.250.4977.99; PMID: 17808240
- Anatskaya OV, Vinogradov AE. Heart and liver as developmental bottlenecks of mammal design: evidence from cell polyploidization. Biol J Linn Soc Lond 2004; 83:175 - 86; http://dx.doi.org/10.1111/j.1095-8312.2004.00377.x
- Lee HO, Davidson JM, Duronio RJ. Endoreplication: polyploidy with purpose. Genes Dev 2009; 23:2461 - 77; http://dx.doi.org/10.1101/gad.1829209; PMID: 19884253
- Simons AM. Modes of response to environmental change and the elusive empirical evidence for bet hedging. Proc R Soc Lond Ser B, Biol Sci 2011; 278:1601 - 9; http://dx.doi.org/10.1098/rspb.2011.0176; PMID: 21411456
- Marler TE. Cycad mutualist offers more than pollen transport. Am J Bot 2010; 97:841 - 5; http://dx.doi.org/10.3732/ajb.0900251; PMID: 21622449
- Gorelick R, Olson K. Is lack of cycad (Cycadales) diversity a result of a lack of polyploidy?. Bot J Linn Soc 2011; 165:156 - 67; http://dx.doi.org/10.1111/j.1095-8339.2010.01103.x
- Gorelick R. Evolution of cacti is largely driven by genetic drift, not selection. Bradleya 2009; 27:41 - 52
- Lynch M, Conery JS. The origins of genome complexity. Science 2003; 302:1401 - 4; http://dx.doi.org/10.1126/science.1089370; PMID: 14631042
- Zonneveld BJM. Genome sizes for all genera of Cycadales. Plant Biol 2012; 14:253 - 6; PMID: 22117644
- Gould SJ, Lewontin RC. Spandrels of San Marco and the Panglosian paradigm: a critique of the adaptationist program. Proc R Soc Lond Ser B, Biol Sci 1979; 205:581 - 98; http://dx.doi.org/10.1098/rspb.1979.0086
- Hoffmann M, Hilton-Taylor C, Angulo A, Böhm M, Brooks TM, Butchart SH, et al. The impact of conservation on the status of the world’s vertebrates. Science 2010; 330:1503 - 9; http://dx.doi.org/10.1126/science.1194442; PMID: 20978281
- Forster PI. Recovery plans for endangered cycads: a model set of objectives and actions using the example of Cycas megacarpa from Queensland, Australia. Mem NY Bot Gard 2007; 97:3 - 31
- Nagalingum NS, Marshall CR, Quental TB, Rai HS, Little DP, Mathews S. Recent synchronous radiation of a living fossil. Science 2011; 334:796 - 9; http://dx.doi.org/10.1126/science.1209926; PMID: 22021670
- Stevenson DW. Morphology and systematics of the Cycadales. Mem NY Bot Gard 1990; 57:8 - 55