Abstract
This paper explores further the “behavioral homeostasis theory” (BHT) regarding the evolutionary significance for organism survival of the two simple non-associative rapidly learned behaviors of habituation and sensitization. The BHT postulates that the evolutionary function of habituation and sensitization throughout phylogeny is to rapidly maximize an organism’s overall readiness to cope with new stimuli and to minimize unnecessary energy expenditure. These behaviors have survived with remarkable similarity throughout phylogeny from aneural protozoa to humans. The concept of “behavioral homeostasis” emphasizes that the homeostatic process is more than just maintaining internal equilibrium in the face of changing internal and external conditions. It emphasizes the rapid internal and external effector system changes that occur to optimize organism readiness to cope with any new external stimulus situation. Truly life-threatening stimuli elicit instinctive behavior such as fight, flee, or hide. If the stimulus is not life-threatening, the organism rapidly learns to adjust to an appropriate level of overall responsiveness over stimulus repetitions. The rapid asymptotic level approached by those who decrease their overall responsiveness to the second stimulus (habituaters) and those who increase their overall responsiveness to an identical second stimulus (sensitizers) not only optimizes readiness to cope with any new stimulus situation but also reduces unnecessary energy expenditure. This paper is based on a retrospective analysis of data from 4 effector system responses to eight repetitive tone stimuli in adult human males. The effector systems include the galvanic skin response, finger pulse volume, muscle frontalis and heart rate. The new information provides the basis for further exploration of the BHT including new predictions and proposed relatively simple experiments to test them.
Background of the Behavioral Homeostasis Theory (BHT)
Throughout phylogeny organisms constantly receive sensory input. However, because of circadian rhythmicity (an approximate 24 h day-night metabolic cycle), as well as other intrinsic and extrinsic factors, they are not always at maximal alertness to pay attention and assess the importance of new stimuli when they occur.Citation1 Thus, it is necessary for the organism to have rapid ways to become optimally alert to a new specific repetitive external stimulus in order to evaluate and cope with it, and also to have rapid ways to minimize responding to less significant repetitive stimuli in order to be able to detect other (possibly more important) stimuli, as well as conserve energy. One hypothesis underlying the “behavioral homeostasis theory” (BHT) of habituation and sensitization is that cyclic rhythms play a key role in modulating sensory thresholds, i.e., alertness level, to a new iterative stimulus at any given point in time. Also, that the level of pre-initial stimulus alertness prior to the first stimulus of a new iterative series is likely to be critical in determining the initial direction of overall behavioral change to the second stimulus, i.e., habituation or sensitization. Thus, an organism which is in a high state of alertness when the first stimulus occurs is likely to be very responsive and, if the stimulus is assessed to be of little significance, rapidly decreases its responsiveness, i.e., habituates to the next stimulus. Likewise, if that same organism is less alert when the first stimulus occurs, it is likely to be much less responsive initially, but rapidly sensitizes, i.e., increases its responsiveness to the next stimulus, (hypothetically, in order to receive more information) and, if the stimulus is then assessed to be insignificant, habituation follows. Thus, habituation here is defined as a decrease in overall responsiveness to the second stimulus of a repetitive series. Although the word sensitization has been used in many ways in the literature, in the BHT it represents an increase in overall responsiveness to an identical second stimulus.Citation2,Citation3 These two simple non-associative learned behaviors have been seen throughout phylogeny from aneural single cell microorganisms, such as the protozoa Spirostomum and Stentor, through all the invertebrate and vertebrate phyla, including humans, with remarkable similarity.Citation3-Citation17
Exploring the Concept of Behavioral Homeostasis and How it Relates to the Traditional Concept of Homeostasis
A major component of the BHT is that it views homeostasis as including more than just maintaining internal equilibrium in an organism facing constantly changing internal and external environmental conditions.Citation3,Citation18-Citation20 Classically, homeostasis has referred to maintaining internal equilibrium, such as body temperature in a mammal, during external changes in temperature. However, as the external temperature increases, the organism may move to a shady area, increase its surface to volume ratio by spreading itself out in order to lose heat, as well as by sweating. If the external temperature decreases, an organism may “huddle” to decrease its surface to volume ratio to conserve heat or increase its metabolic activity, e.g., shivering, to generate more heat. All of these internal metabolic and external behavioral changes, which work to constrain body temperature, enhance survival. Likewise, if the organism faces external stimuli that may be life-threatening, e.g., a predator, there is also likely to be a rapid instinctive reaction in the form of internal metabolic changes as well as rapid overall external behavior changes to prepare the organism to fight, flee or hide.Citation21 It is assumed to be unlikely that the simple learned behaviors of habituation or sensitization would play a large role in such genetically pre-programmed behavior to a life-threatening situation, or to any stimulus essential for survival, such as, for example, detecting a pheromone of a member of the same species.Citation22,Citation23 If a repetitive external stimulus is not alarming, then the responsiveness of the organism can very quickly approach a new “homeostatic level of readiness”, i.e., habituate or sensitize, to an asymptotic level of overall body energy expenditure to the repetitive stimulus that is appropriate for the situation. The BHT postulates that the evolutionary function of habituation and sensitization throughout phylogeny is to rapidly maximize an organism’s overall readiness to cope with new stimuli and to minimize unnecessary energy expenditure.
In all of the above examples the organism must rapidly alter its internal metabolic and external behavioral activity to an appropriate level in order to adapt to changes in the situation. Thus, while the word, homeostasis, traditionally has referred to a maintained metabolic level (such as body temperature), the concept of behavioral homeostasis emphasizes the role of the rapid internal and external effector system changes to maximize readiness to cope with any external stimulus situation as well as to maintain internal metabolic equilibrium. How long any new rapidly achieved metabolic and behavioral level remains in place depends, of course, on the nature of the external stimulus field the organism faces at any given point in time. In humans, the internal metabolic changes primarily involve the autonomic nervous system, while external behavioral changes primarily involve both voluntary and reflex skeletal muscular activity.Footnotea
Measuring Habituation and Sensitization Across Phylogeny
In most experiments both habituation and sensitization refer to measurement of effector system responses to repetitive sensory input. The phrase, effector system, commonly includes muscle contractions and glandular secretions.Citation19 However, it is used very broadly across phylogeny, and includes contractile activity and swimming movement in aneural organisms, motor neuron and interneuron spike frequency changes in both invertebrates and vertebrates, as well as a great variety of body muscular movements and activities, such as a startle response.Citation3,Citation5,Citation7,Citation8,Citation11,Citation15 Traditionally, habituation and sensitization have been defined as initial direction of a group average response change (over the first few trials) to a repetitive stimulus, primarily as a function of stimulus intensity. Weaker stimuli tend to lead to initial group average habituation and stronger stimuli to initial group average sensitization.Citation14 Since habituation is the dominant change seen over many trials to a repetitive stimulus, group averages generally show habituation over these trials. However, if subjects are examined individually (rather than part of a group average), one often sees both initial habituaters and initial sensitizers over the first few trials to the same stimulus.Citation3
The concept of the BHT evolved from studies of habituation of the contractile response in the aneural ciliated protozoan, Spirostomum, to a repetitive vibratory stimulus.Citation5,Citation8,Citation9 When the protozoan data were examined and analyzed, the group average change seen over many trials was habituation. However, when the group was divided into high, medium and low initial responders, it was discovered that high initial responders habituated, low initial responders sensitized and medium responders showed little or no change to the exact same stimulus presented to individual protozoa over the first few repetitive trials. All three sub-groups eventually approached the same asymptotic level.
Although originally collected for other purposes, a previously obtained data set of the human galvanic skin response (GSR) to repetitive shock stimuli was then examined to see how the GSR results over trials in single individuals compared with those seen in single protozoa.Citation3,Footnoteb The results showed that high initial responders habituated to the second stimulus and low initial responders sensitized to an identical second stimulus. It also was observed that, with respect to the GSR, the greater the magnitude of the skin conductance level (SCL) prior to the first stimulus of the series, the greater the magnitude of the initial GSR to the first shock stimulus, and the greater the likelihood of habituation of the GSR to the second shock stimulus. The SCL is a well known correlate of “alertness level” in humans.Citation24Footnotec It also is the first metabolic measure in humans to show circadian rhythmicity. This occurs within the first week of life.Citation1 Thus, those subjects with a pre-first stimulus high SCL (very alert) showed initial GSR habituation, while those with a significantly lower pre-initial stimulus SCL (less alert) showed a much smaller initial GSR to the exact same stimulus and sensitization to the second stimulus. The decrease in magnitude of both the SCL prior to the last stimulus of a repetitive series and the GSR, achieved by habituaters and sensitizers over repeated shock stimuli, eventually approach an asymptotic level. The level reached is hypothesized by the BHT to indicate the significance of the repetitive stimulus to the individual at that point in time. It represents a balance between being hyper- alert and thereby too responsive to a stimulus, which could distract that individual from detecting other significant stimuli, and being hypo-alert and too unresponsive and possibly failing to detect a change in the present stimulus. Thus, according to the BHT, it is hypothesized that either initial habituation or sensitization of the GSR should occur in the same individual to the same repetitive stimulus depending on the pre-initial stimulus SCL and the GSR to the initial stimulus.Citation2,Citation3,Citation13
Methods
The same retrospective data set from which the GSR to repetitive shock trials was examined also included unpublished data from several other effector systems in response to repetitive tone trials.Citation3,Citation25 The stimulus protocol for the original experiment, for which this data set originally was collected, consisted of 17 trials in which a tone stimulus followed each shock stimulus 20 sec later. Inter-trial intervals varied from 50–90 sec. Interspersed among these 17 unpaired shock followed by tone trials, were eight tone-alone trials. It is these eight tone-alone trials that provide the stimuli for the new retrospective data analysis involving multiple effector systems shown in and .
Figure 1. (A) The GSR consists of the difference function derived by subtracting the palmar skin conductance level (SCL) immediately before stimulus onset from the highest palmar skin conductance level (SCL) reached within 10 sec after stimulus onset for each subject on each trial. The SCL is measured in micro-Siemens (µS). This is a well established method for recording the GSR.Citation4,Citation26,Citation30 (Habituaters: n = 40; Sensitizers: n = 6). The standard error of the mean (SEM) is shown on each trial for all four effector systems. (B) The finger pulse volume decrease (FPV) to a stimulus is measured as the difference between the log of the mean micro-liters per stroke of the two pulses just preceding the stimulus minus the log of the mean micro-liters per stroke of the smallest two consecutive pulse waves occurring within 30 sec after the stimulus onset. This difference is a measure of the degree of vasoconstriction for each subject on each trial.Citation30 (Habituaters: n = 21; Sensitizers: n = 20). (C) The muscle frontalis (forehead) potential increase (MF) consists of the difference in magnitude between the average height of several consecutive potentials just prior to the stimulus onset and the average height of the largest consecutive potentials occurring within 30 sec following the stimulus. This difference, post-stimulus magnitude minus pre-stimulus magnitude in micro-volts (ΔμV), constitutes the response measure for each subject on each trial.Citation30 (Habituaters: n = 18. Sensitizers n = 21). (D) The heart rate increase (HR) to the stimulus is measured as the difference between the highest rate (shortest R-R interval within 30 sec after stimulus onset) and the immediate pre-stimulus rate for each subject on each trial.Citation30 (Habituaters: n = 14; Sensitizers: n = 28).
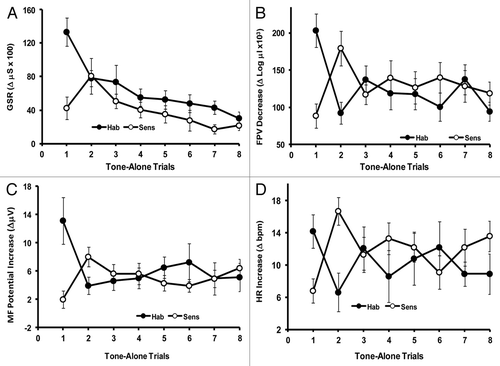
Table 1. Number of subjects showing habituation, sensitization or no change in each effector system from trial 1 to trial 2
Statistical tests
For each effector system, two-tailed paired t-tests were used to compare the mean responses between trials 1 and 2 and between trial 1 and 8 for habituaters and between trials 2 and 3 for sensitizers. Two-tailed unpaired t-tests were used to compare the mean responses for habituaters and sensitizers on trials 1 and 8. A traditional .05 significance level was set for these test statistics. The error bars shown represent the standard error of the mean (SEM) on each trial.
Results
The multiple effector system responses to repetitive tone-alone trials were examined in individuals to see if the results showed any similarity to those seen in individual protozoa over repetitive trials. Interestingly, like the protozoa, and the GSR to repetitive shock, individually examined subjects divided into habituaters (high initial responders) and sensitizers (low initial responders) to the same eight repetitive tone stimuli in each of the four effector systems examined ().
All four effector systems showed considerable similarity in the characteristics of the separated habituation and sensitization curves, respectively:
1. Habituaters, in the four effector systems examined, show a statistically significant decrease in responsiveness from trial 1 to trial 2 as well as between trial 1 and trial 8. Interestingly, as often seen throughout phylogeny, habituaters show their largest ordinal decrease in response magnitude over the first few trials in all four effector systems.
2. Sensitizers, following their increase in responsiveness from trial 1 to trial 2 (which was the basis of their selection), show a dramatic decrease in response magnitude from trial 2 to trial 3 in all four effector systems. The difference from trial 2 to trial 3 was statistically significant in . The difference from trial 2 to trial 3 was not significant for the GSR in . However, the difference from trial 2 to trial 8 was statistically significant for GSR sensitizers. The low number of sensitizers in (n = 6) may account for the lack of statistical significance from trial 2 to trial 3. (See the Discussion section for further speculation on why the number of GSR sensitizers to tone-alone stimuli may be so low).
3. All effector systems show that those who habituate on trial 2 show a statistically significant greater response on trial 1 than those who sensitize from trial 1 to an identical stimulus.
4. When one compares habituater and sensitizer subjects on trial 8, there is no statistically significant difference between them in any of the four effector systems. Habituaters and sensitizers each approach an asymptotic level by trial 8.
It also was observed that apparently the same individual could show initial habituation in one effector system and simultaneously show initial sensitization in another effector system to the same stimulus sequence (see ).
Discussion
An earlier formulation of the BHT postulated that in humans there are two distinct threshold processes invoked initially when a new iterative stimulus series begins, and that these thresholds play a critical role in detecting the stimulus, as well as determining which of these two behavioral changes (habituation or sensitization) initially occurs in a given effector system to the same stimulus set.Citation3,Citation26 They are: (1) the initial level of overall alertness threshold which can be estimated in humans by the baseline palmar skin conductance level (SCL) just prior to the first stimulus of a new stimulus set and (2) the external stimulus input processing threshold to the first stimulus of the set, as estimated by the immediate increase in the SCL, (i.e., the magnitude of change from pre-stimulus SCL to post-stimulus SCL), labeled the galvanic skin response or GSR. The former threshold was postulated to indicate the overall alertness of the person to all stimuli in the environment, while the latter was postulated to indicate the degree of processing of a specific new external stimulus input to determine its significance to the person. (What the BHT labels the external stimulus input processing threshold is often referred to as an ‘orienting reflex’ or ‘focusing of attention’).Citation13
Because the new retrospective analysis of multiple effector systems in the same person showed such variation in the relationship among effector systems in whether they initially habituated or sensitized to the same tone stimulus, the BHT must now consider that habituation and sensitization involve the integration of all the effector systems of a person activated by a new repetitive stimulus, rather than just the initial direction of change in any given effector system. Thus, while habituation and sensitization represent rapid ways to achieve ‘behavioral homeostasis’ in each activated effector system of a person, all effector system changes must be rapidly integrated with each other to achieve a level of “overall behavioral homeostatic readiness” to appropriately respond to novel or redundant stimuli while minimizing energy expenditure.Citation20
Thus, the BHT now speculates that higher organisms have evolved specialized brain functions to govern both the pre-initial level of alertness (measured by the SCL) as well as stimulus significance (measured by the GSR). In addition, it is these same “higher brain functions” that are now hypothesized to control the “integration” of all the effector systems activated by a new stimulus series, and that these same higher brain functions also determine the initial direction of “overall body energy expenditure change” from trial 1 to trial 2, i.e., initial habituation or sensitization.Footnoted
An important question that remains is: How does one measure the overall body energy expenditure of the integrated effector systems which have been activated by the stimulus on trial 1, as well as the change in such energy expenditure over the first two trials? A useful biomarker might be to measure electromyographic (EMG) changes in several major skeletal muscles of the body. An increase in muscle potential means an increase in contraction and, therefore, an increase in energy expenditure. Thus, EMG measures of bilateral flexor and extensor muscles of the upper and lower arms and legs could serve as biomarkers of “overall body energy expenditure” to each repetitive stimulus. The increase in muscle potential change to a new stimulus, when integrated over a variety of major skeletal muscles, may be a simple index (not the total measure) of overall body energy expenditure to that stimulus. Another possible biomarker to reflect overall body energy expenditure change over trials is that of oxygen consumption in the person on each trial. An instantaneous increase in oxygen consumption on a given trial reflects an increase in overall body energy expenditure. The best index reflecting overall body energy expenditure to any stimulus, however, needs to be determined. This technology is currently evolving rapidly in medical research.Citation28
Some new speculative predictions and proposed tests of them
There are 3 new predictions, based on the new retrospective findings, which can be tested initially on human subjects:
1. On trial 1, GSR habituater subjects will show much greater integrated overall body energy expenditure to the initial stimulus than GSR sensitizer subjects. On trial 2, GSR habituater subjects will show a large decrease, and sensitizer subjects a large increase, in integrated overall body energy expenditure in response to the second stimulus. Therefore, both measures—GSR effector system change and overall body energy expenditure change—over the first two trials are predicted to change in the same direction for each individual.
2. Those individual subjects who show no GSR change from trial 1 to trial 2 will show no change in overall body energy expenditure from trial 1 to trial 2 because (it is hypothesized) they are already at an optimal state of “alertness/readiness” to cope with the current stimulus situation.
3. If any of the effector system measurements, other than the GSR, are used to test the above 2 predictions in humans, (e.g., finger pulse volume, muscle frontalis, heart rate), it is unlikely there will be a consistent relationship between the initial direction of effector system change in any one of these other effector systems (habituation or sensitization) and the initial direction of integrated overall body energy expenditure change in the same individual from trial 1 to trial 2, i.e., habituation or sensitization. This predicted lack of correlation is based on the new retrospective finding that the level of “overall alertness” prior to the first tone-alone stimulus, as measured by the SCL, was found to be significantly greater in habituater vs. sensitizer subjects with respect to the GSR [for both tone () and shock stimuli,Citation3], but not significantly different prior to the first tone-alone stimulus between habituater and sensitizer subjects in any of the other effector systems (). This suggests that the SCL per se is unlikely to be a major factor in determining whether any specific effector system (other than the GSR) in humans shows initial habituation or sensitization.
Another possible test to consider (based on a prediction from a previous paper on the BHT) is running two trials only when a given subject is at the height of his/her circadian alertness (maximum resting SCL), and to repeat these same two trials some time later when the same subject is at his/her lowest level of circadian alertness (minimum resting SCL).Citation2 Conversely, some subjects would start when at their lowest resting SCL. The prediction from the BHT is that a subject, if at his/her maximum resting SCL when the initial stimulus occurred, would show GSR habituation to the second stimulus if assessed as of little significance. Likewise, the same subject would show GSR sensitization to the same new stimulus if it occurred when at his/her minimum resting SCL. Such a study could provide additional support for the BHT.
In it is interesting that the number of GSR sensitizers was quite low compared with the other three effector systems. The retrospective data used for this analysis consisted of both shock trials and tone trials. Since the initial trial was a shock trial, it is likely that this would be far more “alerting” to the individual than a tone trial. Accordingly, one speculative idea, based on the BHT, is that the initial shock trial had a strong “alerting effect”. Thus, when the first tone-alone trial occurred, individuals were much more alert to assess it, and were more likely to habituate than sensitize. In support of this speculation, the number of GSR habituater and sensitizer subjects to the initial shock trial was 28 and 17, respectively,Citation3 and to the first tone-alone trial 40 and 6, respectively. Further, there was a marked increase in pre-initial stimulus SCL (alertness) for habituaters prior to the first tone-alone trial compared with pre-initial alertness for habituaters prior to the first shock trial. In addition, as noted in prediction 3, the level of alertness prior to the first tone-alone stimulus was significantly greater for habituater than sensitizer subjects with respect to the GSR, but not significantly greater prior to the first tone-alone stimulus between habituater and sensitizer subjects in any of the other effectors. This is only a hypothetical interpretation. In the future, to test the theory properly requires that only one repetitive stimulus, e.g., a tone, be used.
To summarize, it is now speculated that all effector systems in a given person, activated by a new non-life-threatening stimulus set, must be rapidly integrated to maximize “overall readiness” to cope with the new stimulus and minimize body energy wastage. The factors that control the specific initial direction of responsiveness change to a repetitive stimulus (habituation or sensitization) for each of the individual body effector systems activated (other than those hypothesized for the GSR) are yet to be determined. However, factors such as (a) the nature of the stimulus, (b) the metabolic level any given effector system is at when the new stimulus occurs, (c) the type of effector system, as well as its innervation, may be important.
Simple experiments can be conducted with human participants to test the new predictions as well as past predictions.Citation2,Citation3 Tests of these predictions are based on measuring individual human subject relationships between the direction of a given effector system change, such as the GSR, from trial 1 to trial 2 (habituation or sensitization) and the direction of the integrated overall body energy expenditure change (habituation or sensitization) from trial 1 to trial 2. Most organisms across phylogeny do not have SCL and GSR indices that are hypothesized to reflect the higher brain functions associated, respectively, with “initial pre-stimulus alertness” and “stimulus processing” of a novel stimulus. In many cases, however, α rhythm (8–12 Hz EEG oscillations) may serve as an index of brain alertness, as well as a change in brain alertness, over a variety of complex organisms such as mammals.Citation24,Citation29 At lower phylogenetic levels, measures such as overall organism activity may be a useful index of alertness level. This needs to be explored further in order to fully test the BHT across the phylogenetic spectrum.
Conclusions
The two simple non-associative learned behaviors of habituation and sensitization undoubtedly evolved by both homologous and analogous (convergent) evolutionary processes. The fact that they are seen with such remarkable similarity from protozoa to humans strongly indicates their importance for survival. The processes or mechanisms underlying these two behaviors may vary within the same organism in different effector systems, as well as across phylogeny. However, the marked similarity of the two behaviors across phylogeny speaks to the uniformity of their function which, according to the BHT, is to allow the organism to rapidly optimize its readiness to cope with any new stimulus situation and, at the same time, minimize wastage of body energy.Citation20
| Abbreviations: | ||
| BHT | = | behavioral homeostasis theory |
| SCL | = | palmar skin conductance level |
| GSR | = | galvanic skin response |
| SCN | = | suprachiasmatic nucleus of the hypothalamus |
| EMG | = | electromyogram |
| FPV | = | finger pulse volume |
| MF | = | muscle frontalis |
| HR | = | heart rate |
| EEG | = | electroencephalogram |
| SEM | = | standard error of the mean |
Acknowledgments
The senior author is greatly indebted to the late Professor E.N. Sokolov of Moscow State Lomonosov University for his insightful and supportive comments and thoughts on the original “BHT”. I would like to thank Prof. R. Reep of the University of Florida and his colleague, Prof. G. Bauer, of the New College of Florida for their helpful comments. I wish to thank the anonymous reviewers for their insightful comments and suggestions which greatly improved this paper. I also would like to thank the VA Greater Los Angeles Health Care System for its support in the preparation of this article.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Notes
a A recent paper on neuronal homeostasisCitation20 notes that “homeostatic mechanisms fit into a hierarchy which operates on the levels of single proteins, protein networks, whole cells, cellular networks, organs and ultimately entire organisms.” This article is very relevant to the BHT.
b The galvanic skin response (GSR) consists of the difference function derived by subtracting the palmar skin conductance level (SCL) immediately before stimulus onset from the highest skin conductance level (SCL) reached within 10 sec after stimulus onset for each subject on each trial.
c Many words have been used to signify what we term “alertness level”. They include “awareness”, “vigilance”, “activity level”, “detection”, “wakefulness”, and “readiness”. In this paper, when discussing human responsiveness to a new stimulus, we use the term “alertness level” as measured by the SCL prior to the new stimulus.
d A recently published paper on circadian rhythmicity in mammals suggests that the suprachiasmatic nucleus (SCN) in the hypothalamus functions as the body’s “master clock.” It synchronizes all of the body’s peripheral clocks. Thus, such a higher brain center or “master clock” could play a major role in determining the “initial direction of overall body energy expenditure change” of the combined body effector systems, i.e., habituation or sensitization from trial 1 to trial 2 in mammals, depending on the phase of the circadian cycle, i.e., the “level of alertness,” at the start of a new repetitive stimulus.Citation27
References
- Conroy RTWL, Mills JN. Human circadian rhythms. London: J. & A. Churchill, 1970.
- Eisenstein EM, Eisenstein D, Smith JC. The evolutionary significance of habituation and sensitization across phylogeny: A behavioral homeostasis model. Integr Physiol Behav Sci 2001; 36:251 - 65; http://dx.doi.org/10.1007/BF02688794
- Eisenstein EM, Eisenstein D. A behavioral homeostasis theory of habituation and sensitization: II. Further developments and predictions. Rev Neurosci 2006; 17:533 - 58; http://dx.doi.org/10.1515/REVNEURO.2006.17.5.533; PMID: 17180878
- Boucsein W. Electrodermal activity. New York: Plenum Press, 1992.
- Eisenstein EM, Brunder DG, Blair HJ. Habituation and sensitization in an aneural cell: some comparative and theoretical considerations. Neurosci Biobehav Rev 1982; 6:183 - 94; http://dx.doi.org/10.1016/0149-7634(82)90054-9; PMID: 6285234
- Eisenstein EM, Peretz B. Comparative aspects of habituation in invertebrates. In: Peeke, H.V.S. Herz, M.J., eds. Habituation. Physiological substrates, V2. New York & London: Academic Press, 1973: 1-34.
- Groves PM, Thompson RF. A dual-process theory of habituation: Neural mechanisms. In: Peeke, H.V.S. Herz, M.J., eds. Habituation: Physiological substrates. V2. New York & London: Academic Press, 1973: 175-205.
- Hamilton TC, Thompson JM, Eisenstein EM. Quantitative analysis of ciliary and contractile responses during habituation training in Spirostomum ambiguum. J. Behav. Biol. 1974; 12:393 - 407; http://dx.doi.org/10.1016/S0091-6773(74)91601-0; PMID: 4217176
- Hamilton TC. Behavioral plasticity in protozoans. In: Eisenstein, E.M., ed. Aneural organisms in neurobiology. New York: Plenum Press, 1975: 111-130.
- Kandel ER. Cellular basis of behavior. San Francisco: W.H. Freeman & Co, 1976.
- Pakula A, Sokolov EN. Habituation in Gastropoda: Behavioral, interneuronal, and endoneuronal aspects. In: Peeke, H.V.S., Herz, M.J., eds. Habituation: Physiological substrates. V2. New York & London: Academic Press, 1973: 35-107.
- Rankin CH, Abrams T, Barry RJ, Bhatnagar S, Clayton DF, Colombo J, et al. Habituation revisited: an updated and revised description of the behavioral characteristics of habituation. Neurobiol Learn Mem 2009; 92:135 - 8; http://dx.doi.org/10.1016/j.nlm.2008.09.012; PMID: 18854219
- Sokolov EN, Spinks JA, Lyytinen H. The orienting response in information processing. Hillsdale, N.J.: Lawrence Erlbaum Assoc., 2002.
- Thompson RF, Berry SD, Rinaldi PC, Berger TW. Habituation and the orienting reflex: The dual-process theory revisited. In: International Conference on Orienting Reflex in Humans. Hillsdale, New Jersey: Lawrence Erlbaum Assoc., 1979: 21-60.
- Thompson RF, Groves PM, Tyler TJ, Roemer RA. A dual-process theory of habituation: Theory and behavior. In: Peeke, H.V.S., Herz, M., eds. Habituation: Behavioral studies, V1. New York & London: Academic Press, 1973: 239-271.
- Wood DC. Electrophysiological correlates of the response decrement produced by mechanical stimuli in the protozoan, Stentor coeruleus. J Neurobiol 1970; 2:1 - 11; http://dx.doi.org/10.1002/neu.480020102; PMID: 4333386
- Wood DC. Parametric studies of the response decrement produced by mechanical stimuli in the protozoan, Stentor coeruleus. J Neurobiol 1970; 1:345 - 60; http://dx.doi.org/10.1002/neu.480010309; PMID: 5407046
- Cannon WB. The Wisdom of the Body. New York: Norton, 1939.
- Taber’s Cyclopedic Medical Dictionary. 18th Edition. Phil., PA: FA Davis Co., 1997.
- O’Leary T, Wyllie DJ. Neuronal homeostasis: time for a change?. J Physiol 2011; 589:4811 - 26; PMID: 21825033
- Brudzynski SM. Pharmacological and behavioral characteristics of 22 kHz alarm calls in rats. Neurosci Biobehav Rev 2001; 25:611 - 7; http://dx.doi.org/10.1016/S0149-7634(01)00058-6; PMID: 11801286
- Holy TE, Dulac C, Meister M. Responses of vomeronasal neurons to natural stimuli. Science 2000; 289:1569 - 71; http://dx.doi.org/10.1126/science.289.5484.1569; PMID: 10968796
- Schleidt W, Shalter MD, Moura-Neto H. The hawk/goose story: the classical ethological experiments of Lorenz and Tinbergen, revisited. J Comp Psychol 2011; 125:121 - 33; http://dx.doi.org/10.1037/a0022068; PMID: 21341906
- Davies DR, Krkovic A. Skin-conductance, alpha-activity, and vigilance. Am J Psychol 1965; 78:304 - 6; http://dx.doi.org/10.2307/1420507; PMID: 14290763
- Eisenstein EM, Eisenstein D, Bonheim P. Initial habituation or sensitization of the GSR depends on magnitude of first response. Physiol Behav 1991; 49:211 - 15; http://dx.doi.org/10.1016/0031-9384(91)90256-N; PMID: 2017477
- Lykken DT, Venables PH. Direct measurement of skin conductance: a proposal for standardization. Psychophysiology 1971; 8:656 - 72; http://dx.doi.org/10.1111/j.1469-8986.1971.tb00501.x; PMID: 5116830
- Buhr ED, Yoo S-H, Takahashi JS. Temperature as a universal resetting cue for mammalian circadian oscillators. Science 2010; 330:379 - 85; http://dx.doi.org/10.1126/science.1195262; PMID: 20947768
- Kim D-H, Lu N, Ma R, Kim Y-S, Kim R-H, Wang S, et al. Epidermal electronics. Science 2011; 333:838 - 43; http://dx.doi.org/10.1126/science.1206157; PMID: 21836009
- McKinney SM, Dang-Vu TT, Buxton OM, Solet JM, Ellenbogen JM. Covert waking brain activity reveals instantaneous sleep depth. PLoS One 2011; 6:e17351; http://dx.doi.org/10.1371/journal.pone.0017351; PMID: 21408616
- Wenger MA, Engel BT, Clemens TL. Studies of autonomic response patterns: Rationale and methods. Behav Sci 1957; 2:216 - 21; http://dx.doi.org/10.1002/bs.3830020304