Abstract
This paper expands on recent findings that link dynamic patterns of striatal activity with patterns of movement and exploration in wild-type and transgenic mice (R6/2) that model Huntington disease (HD), a fatally inherited neurological condition. Here, with HD as a backdrop, we further develop the concept of entropy conservation in brain and behavior. In particular, we propose that entropy conservation could serve as a rule that guides the process of redistributing brain activity dynamics in order to alter behavior, allowing the adaptation to an ever-changing external environment. This concept is further linked to recent neuroimaging studies in human aging, building a new bridge between our recent findings of entropy conservation and the extant literature.
Neural Correlates of Unpredictability in Behavioral Patterns
A common feature of Huntington disease (HD), an autosomal dominant condition, is a severe loss of neurons in striatum,Citation1 the main information processing unit of the basal ganglia.Citation2 Ample evidence obtained from transgenic mice that model HD indicates that prior to the death of these neurons, they become dysfunctional and no longer process information in the same way that healthy ones do.Citation3,Citation4 At the level of behavior, HD is typically observed as a decline in motor control sometimes called “chorea” for the dancelike movements that result. In R6/2 mice, which express the toxic fragment of the HD gene (exon 1), a prominent behavioral phenotype is motor inflexibility, which is manifest as a decrease in the number of turns made into the perpendicular arms of a plus-shaped maze.Citation5 Our recent studyCitation6 sought to gain further insight into the underlying neural correlates of this phenotype. We specifically targeted the striatum, the primary “decision-making” region of the basal ganglia that integrates cognitive and sensorimotor input from cerebral cortex in order to select and execute patterns of movement.Citation7 We examined whether striatal activity, as measured by local field potentials (LFPs), are associated with motor inflexibility observed in HD mice.
To examine behavior-related patterns of LFP activity, we turned to concepts based on probabilities in order to measure the likelihood of turning in a plus-shaped maze and the pattern of maze exploration. Using an event-related approach, we examined the patterns of striatal LFP activity around the point in time at which the mice crossed the choice-point in the center of the maze as well as 2 sec before and 2 sec after they made the choice-point crossing. Although the striatal LFPs of the HD mice were significantly more unpredictable than their wild-type (WT) controls at all time-points, the HD LFPs exhibited a similar pattern of change over time. Entropy in the striatal LFPs of both types of mice decreased as they entered the choice point and increased as they departed from the choice point into an arm of the maze (forming a U-shape). Interestingly, we observed that more unpredictable LFP dynamics were correlated with more predictable patterns of behavior, as measured through: (1) the distribution of arm-choices; (2) likelihood of running straight along the maze; and (3) likelihood of performing alternating choice decisions (i.e., turning 90° then running straight and vice versa).
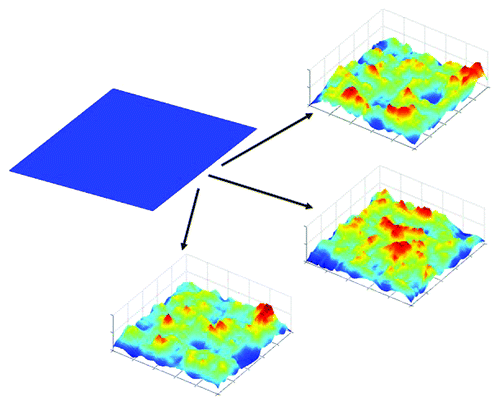
These findings support the entropy conservation hypothesis (illustrated in ), which predicts that total entropy in neural activity across the brain is held constant, and is consistently being redistributed across the entire brain in order to respond to changes in the external environment.Citation8,Citation9 Because it is impossible to measure activity in all brain neurons, entropy of the behavioral decision patterns of the mice serves as a proxy for the collective entropy of activity in the brain regions that we could not measure. Under this assumption, lower levels of entropy in striatal LFP activity reflect situations in which entropy has been “passed along” to other regions of the brain. The ability of the remaining brain regions to “receive” entropy from the striatum is potentially what allows greater flexibility in the motor performance of WT mice. It is conceivable that the already high level of entropy in the striatal LFP signal of R6/2 mice prevented the necessary increase in entropy of the other brain regions to increase motor flexibility. Because the entropy in the HD mouse striatum remained high across all the time points around the choice-point crossing, the entropy in all the other regions responsible for movement and exploration would have to be lower, as reflected in the more predictable patterns of behavioral dynamics and exploration.
Figure 1. Schematic illustration of the concept of entropy conservation in the brain as a process similar to origami. Imagine that total brain entropy is represented as a piece of paper that can be folded to form landscapes with peaks of different heights and valleys of different depths. However, the total amount of available material is limited and thus, must be redistributed. When new peaks are created in one area, valleys must be created in another.
Our recent findingsCitation6 also are consistent with functional magnetic resonance imaging (fMRI) studies of human aging. Especially relevant is the finding of compensatory brain activation in healthy aging. It has been shown that older individuals who are able to activate non-motor areas of the brain perform better than older people who simply engage motor regions.Citation10 The same study found that the seniors who performed poorly on the motor task engaged similar brain regions as the young subjects (who outperformed all of the older subjects). These poorly performing seniors exhibited an inability to activate supplementary brain regions to compensate for age-related motor declines. In a similar vein, a recent study of cognitive performance in aging showed that higher variability in the blood oxygenation-level-dependent (BOLD) signal in the brain is associated with higher performance.Citation11 Higher BOLD signal variability, however, was not observed across the entire brain; subcortical regions were nearly an order of magnitude less variable than all the other regions in which BOLD signal variability was high. In contrast, the individuals that performed poorly on the cognitive task exhibited moderate levels of variability across all brain regions. Based on the brain regions in which variability was high, it was suggested that these were the task-relevant areas that were most heavily involved in cognitive performance.
If greater variability is equated to higher entropy or greater signal unpredictability, these human studies mirror our current findings, and could be considered further support for the entropy conservation hypothesis in brainCitation8,Citation9 and behavior.Citation12,Citation13 Similar to our findingsCitation6 that suggest motor inflexibility in HD arises because of an aberrant redistribution of signal unpredictability across the brain, cognitive deficits in the elderly might also be associated with a dysfunctional redistribution of entropy across the brain. Such an idea is consistent with the view that “neural noise” or unpredictable brain activity serves a functional purpose in neural network configurations.Citation14,Citation15 In conclusion, the synthesis of these findings in human subjects and our recent data indicate that behavioral patterns are altered through the shifting of entropy to different regions across the brain. Additionally, this process of brain activity redistribution occurs in a manner that conserves entropy, acting as a form of natural law that governs the modulation of brain activity across different behaviors.
Acknowledgments
This work was supported by the National Institute on Aging R21 AG 039818 and R21 AG 035158 and National Institute of Neurological Diseases and Stroke R01 NS 35663.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Vonsattel JPG, DiFiglia M. Huntington disease. J Neuropathol Exp Neurol 1998; 57:369 - 84; http://dx.doi.org/10.1097/00005072-199805000-00001; PMID: 9596408
- Bolam JP, Hanley JJ, Booth PAC, Bevan MD. Synaptic organisation of the basal ganglia. J Anat 2000; 196:527 - 42; http://dx.doi.org/10.1046/j.1469-7580.2000.19640527.x; PMID: 10923985
- Miller BR, Walker AG, Shah AS, Barton SJ, Rebec GV. Dysregulated information processing by medium spiny neurons in striatum of freely behaving mouse models of Huntington’s disease. J Neurophysiol 2008; 100:2205 - 16; http://dx.doi.org/10.1152/jn.90606.2008; PMID: 18667541
- Raymond LA, André VM, Cepeda C, Gladding CM, Milnerwood AJ, Levine MS. Pathophysiology of Huntington’s disease: time-dependent alterations in synaptic and receptor function. Neuroscience 2011; 198:252 - 73; http://dx.doi.org/10.1016/j.neuroscience.2011.08.052; PMID: 21907762
- Rebec GV, Barton SJ, Marseilles AM, Collins K. Ascorbate treatment attenuates the Huntington behavioral phenotype in mice. Neuroreport 2003; 14:1263 - 5; http://dx.doi.org/10.1097/00001756-200307010-00015; PMID: 12824772
- Hong SL, Barton SJ, Rebec GV. Altered Neural and Behavioral Dynamics in Huntington’s Disease: An Entropy Conservation Approach. PLoS One 2012; 7:e30879; http://dx.doi.org/10.1371/journal.pone.0030879; PMID: 22292068
- Redgrave P, Rodriguez M, Smith Y, Rodriguez-Oroz MC, Lehericy S, Bergman H, et al. Goal-directed and habitual control in the basal ganglia: implications for Parkinson’s disease. Nat Rev Neurosci 2010; 11:760 - 72; http://dx.doi.org/10.1038/nrn2915; PMID: 20944662
- Mandell AJ, Selz KA. Entropy conservation as Tμ≈λ+dμ in neurobiological dynamical systems. Chaos 1997; 7:67 - 81; http://dx.doi.org/10.1063/1.166241; PMID: 12779638
- Smotherman WP, Selz KA, Mandell AJ. Dynamical entropy is conserved during cocaine-induced changes in fetal rat motor patterns. Psychoneuroendocrinology 1996; 21:173 - 87; http://dx.doi.org/10.1016/0306-4530(95)00040-2; PMID: 8774061
- Heuninckx S, Wenderoth N, Swinnen SP. Systems neuroplasticity in the aging brain: recruiting additional neural resources for successful motor performance in elderly persons. J Neurosci 2008; 28:91 - 9; http://dx.doi.org/10.1523/JNEUROSCI.3300-07.2008; PMID: 18171926
- Garrett DD, Kovacevic N, McIntosh AR, Grady CL. The importance of being variable. J Neurosci 2011; 31:4496 - 503; http://dx.doi.org/10.1523/JNEUROSCI.5641-10.2011; PMID: 21430150
- Hong SL. The entropy conservation principle: applications in ergonomics and human factors. Nonlinear Dynamics Psychol Life Sci 2010; 14:291 - 315; PMID: 20587303
- Hong SL, Newell KM. Entropy conservation in the control of human action. Nonlinear Dynamics Psychol Life Sci 2008; 12:163 - 90; PMID: 18384715
- Breakspear M, McIntosh AR. Networks, noise and models: reconceptualizing the brain as a complex, distributed system. Neuroimage 2011; 58:293 - 5; http://dx.doi.org/10.1016/j.neuroimage.2011.03.056; PMID: 21447393
- McIntosh AR, Kovacevic N, Lippe S, Garrett D, Grady C, Jirsa V. The development of a noisy brain. Arch Ital Biol 2010; 148:323 - 37; PMID: 21175017