Abstract
Cdc42 is a key factor in the control of cell polarity and morphogenesis. Fission yeast Cdc42 regulates formin activation and actin cable assembly. Cdc42 is also required for exocyst function, contributing to polarized secretion. Additionally, Cdc42 participates in membrane trafficking, endosome recycling, and vacuole formation. We show here how Cdc42 is required for the correct transport/recycling to the plasma membrane of the glucan synthases Bgs1 and Bgs4, responsible of cell wall biosynthesis and polarized growth at the cell tips.
In a vast majority of eukaryotic organisms, Rho GTPase Cdc42 orchestrates the establishment of polarized growth by organizing a number of processes that include actin cytoskeleton organization, polarized secretion or endocytosis. Dissection of these functions is not an easy task due to the close relationship among these processes.
Polarized secretion requires actin cables, which function as tracks for myosin V motors, in order to carry post-Golgi secretory vesicles toward the sites of polarized growth. In the vicinity of the plasma membrane, the octameric complex called exocyst will serve as a tether to keep the vesicle in close relationship with the plasma membrane and facilitating the membrane fusion by the formation of the SNARE complex prior to the release of the vesicle cargo.Citation1 Thanks to the characterization of two novel Schizosaccharomyces pombe cdc42 thermosensitive mutant strains that lack functional actin cables, our lab unveiled the role of Cdc42 and the multidomain scaffold protein Pob1 in the activation of the formin For3, which generates the interphase actin cables.Citation2,Citation3 Interestingly, for3Δ mutant strain lacks actin cables but did not show major secretion defects.Citation4,Citation5 Combination of for3 deletion with exocyst defective cells resulted in non-polarized cells.Citation4 Based on these results it was concluded that actin cables and the exocyst complex collaborate in polarized growth in Schizosaccharomyces pombe, and both are regulated by Cdc42.Citation4 On the other hand, in S. cerevisiae, Cdc42 has been shown to be transported to the plasma membrane along the secretory pathway.Citation6 Although there is no direct demonstration for this in S. pombe, a defect in the secretory machinery might lead to a reduced amount of Cdc42 in the plasma membrane. As a consequence, polarized secretion would not be activated by Cdc42, leading to even more reduced Cdc42 levels in the plasma membrane. In a recent study,Citation7 it has been shown that Cdc42 localization in budding yeast requires phosphatydilserine (PS). The targetting of PS to the plasma membrane is achieved though the secretory pathway. Reduced secretion rates would lead to reduced PS levels in the plasma membrane, resulting in the retention of Cdc42 in endomembranes.
We recently described that the cdc42L160S mutant strain has defects in secretion and vesicle trafficking which are independent of the actin defects.Citation5 Moreover, we and others showed that the scaffold protein Pob1 also shows defects in secretion, suggesting that Pob1 might be involved in the regulation of other Cdc42 functions besides For3 activation. The exocyst localization to the cell tips is lost in cdc42L160S or pob1–664 mutant strains in fission yeast.Citation5,Citation8 How Cdc42 and Pob1 regulate the exocyst function? It has been proposed that the interaction of Rho GTPases might relieve an intermolecular interaction between exocyst subunits that would lead to its activation.Citation9 Other members of the Rho family of proteins, like Rho3, regulate secretion in budding yeast. Thus, Rho3 interacts with the exocyst subunit Exo70.Citation10,Citation11 Moreover, the mutant strain rho3-V51, shows specific secretion defects, though no exocyst deslocalization is observed, suggesting a lack of proper exocyst activation rather than a localization problem.Citation12 In fission yeast Rho3 was found to partially suppress the exocyst mutant sec8–1.Citation13 Additionally, rho3+ overexpression restores the exocyst localization in cdc42L160S or pob1–664, suggesting that both Rho3 and Cdc42-Pob1 play a direct role in targeting the exocyst to the growth sites. Genetic data suggest that Rho3 might function independently to Cdc42. However, the actual relationship between Cdc42 and Rho3 in the regulation of the polarized secretion remains to be elucidated. In budding yeast it seems that the relationship between Cdc42 and Rho3 regulating exocytosis has to do with the phase of the cell cycle. In this case, Cdc42 would be essential in the activation of secretion during bud emergence and apical growthCitation14 whereas Rho3 might work in all phases of the cell cycle.Citation10 However, the pattern of growth of S. pombe does not discriminate these stages, being difficult to imagine a sequential mode of action. Additional experiments will be necessary to decipher this issue. The fact that Rho3 overexpression restores the secretion defect and the exocyst localization, but not the actin cables or the proper morphology of cdc42L160S cells also raises the possibility that actin cytoskeleton becomes essential for achieving the correct morphology in fission yeast. Rho3 might enhance nonpolarized secretion, and causing an increase of plasma membrane PIP2. This phosphoinositide has been shown to play a role in the localization of the exocyst subunit Exo70.Citation1
Biochemical data showed that active GTP-bound Cdc42 levels are increased in cdc42L160S cells compared with a wild type strain. However, this active GTPase is not properly localized to the cell periphery, becoming more concentrated in endomembranes. Overproduction of the Cdc42 GEFs in fission yeast, Gef1 and Scd1, rescues the growth defect of cdc42L160S cells at high temperature. Since both activators are localized to the cell periphery,Citation15-Citation17 we can assume that the suppression of this phenotype takes place mainly through the activation of the inactive Cdc42L160S present in the plasma membrane. These results also suggest that the pool of Cdc42 anchored in the endomembrane compartments is not able to activate secretion.
An additional defect detected in cdc42L160S cells is the drastic fragmentation of the vacuoles and accumulation of endosomes labeled with Syb1, a v-SNARE.Citation18 This defect might be due to the abnormal accumulation of active Cdc42-GTP in the endomembranes. The vacuole fragmentation is suppressed by overexpression of Pob1, Rho3, and the t-SNARE Psy1.Citation19 In all these cases, there is an improvement in Syb1 localization. Rho3 has been proposed to increase the Golgi to endosome trafficking.Citation20 This traffic might also contribute to the reduction of the post-Golgi vesicles accumulation. It will be interesting to show if Cdc42 directly controls this vesicle pathway.
Numerous subcellular localizations have been shown for Cdc42 including Golgi and vacuole membrane where it controls actin polymerization activity during homotypic vacuole fusion.Citation21 In S. cerevisiae it has been shown that actin disassembly is an early subreaction of vacuole membrane fusion, whereas the formation of F-actin is needed for fusion and is likely catalyzed by components activated by Cdc42 at the membrane surface.Citation22,Citation23 In this yeast, overexpression of either constitutively active or dominant-negative Cdc42 resulted in vacuole fragmentation.Citation21 It is likely that multiple vesicle transport and fusion processes include a Cdc42-regulated actin remodeling subreaction, and the hyperactive Cdc42L160S concentrated in endomembranes might block these processes.
Membrane traffic defects might cause cell integrity defects. Indeed, cdc42L160S cells show a mild cell lysis phenotype, and the thermosensitive growth defect can be suppressed by the presence of an osmotic stabilizer such as sorbitol 1.2M (). Sorbitol addition can also alleviate endocytosis defects associated with plasma membrane internalization.Citation24 However, we did not see any defects in the first stages of FM4–64 internalization in cdc42L160S cells.Citation5 Therefore we performed cell wall analysis and showed that these cells have a defective cell wall at 36°C with a significant decrease in (1,3)β-D-glucan, the main structural polymer. Whereas in wild-type cells this polymer represented 20.2 ± 1.2% of total glucose incorporated at 28°C and 24.2 ± 0.8% at 36°C, in cdc42L160S cells corresponded to 19.0 ± 3.2% of total glucose incorporated at 28°C and 16.1 ± 3.8 at 36°C (). (1,3)β-D-glucan is synthesized by Bgs1 and Bgs4 two integral membrane proteins that form part of the (1,3)β-D-glucan synthase. Bgs1 is responsible for the synthesis of the specific (1,3)β-D-glucan that constitutes the fission yeast primary septum and also synthesizes part of the (1,3)β-D-glucan at the poles during apical growth.Citation25,Citation26 Bgs4 synthesizes most of the β-D-glucan that forms the cell wall during growth.Citation27 Both Bgs proteins can concentrate to cell tips in absence of either actin cables or exocyst but not in absence of both. Although F-actin actin is dispensable for their maintenance at the growing sites as part of the plasma membrane, they need actin polymerization for their internalization from the plasma membrane.Citation25-Citation27 Once internalized and delivered to early endosomes they may follow two different routes: recycling to the Golgi and plasma membrane or maturation to late endosomes, ultimately delivered to lisosomes/vacuoles where their contents are degraded.
Figure 1. (A) Growth of wild-type (wt) and cdc42L160S cells spotted at an A600 of 1.0 and 1/4 dilutions in rich medium without or with 1.2M sorbitol. Plates were incubated at different temperatures for 3 d. (B) Cell wall composition of wild-type (wt) and cdc42L160S strains. Cells were incubated at 28°C or 36°C and 14C-glucose was added 6 h before harvesting the cells. Values are the mean of three independent experiments with duplicate samples. Error bars represent standard deviations for the total carbohydrate values. (C) Fluorescence images of GFP-Bgs1 and GFP-Bgs4 in wild-type (wt) and cdc42L160S cells grown at 32°C. (D) Recovery of GFP-Bgs4 in wild-type and cdc42L160S cells. Photo-bleaching of a small region at the tip was accomplished using a Delta Vision system by applying 488-nm laser to cell tips. Images were acquired prior to photobleaching, immediately after and subsequently at regular 10 sec intervals. Left panel shows the average recovery of GFP-Bgs4 (n = 10) in this experiment. (E) Photo-bleaching of the entire cell tip was accomplished using a Leica confocal system by applying 488-nm laser to a region of 40x10 pixels. Images were acquired prior to photobleaching, immediately after and subsequently at regular 1 min intervals.
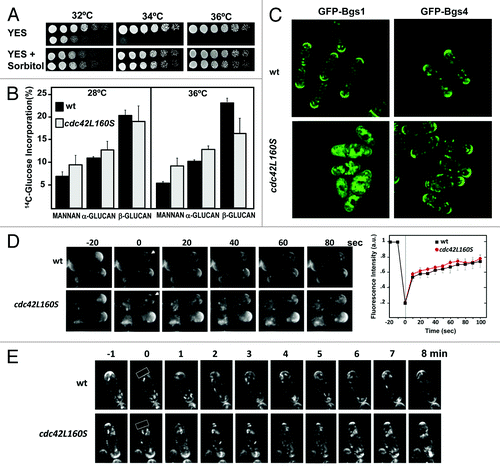
To see if the cdc42L160S cell wall defects were due to mislocalization of Bgs1 or Bgs4, we analized the localization of these proteins labeled with GFP at semi-permissive temperature (32°C). As shown in , GFP-Bgs1 and GFP-Bgs4 were localized to the tip and to the division area of wild-type cells. GFP-Bgs4 had a similar location in cdc42L160S cells. Moreover, fluorescence recovery after photobleaching (FRAP) experiments in which we photobleached GFP-Bgs4 signal from the cell tip of wild-type or cdc42L160S cells, showed no significant difference in the rate of recovery, either if we bleached a small tip area () or if we bleached the whole cell tip (). These results suggested no major defects in membrane diffusion and traffic of Bgs4, although some internal accumulation was observed in cdc42L160S cells. In contrast, GFP-Bgs1 localized very poorly at the tips of cdc42L160S cells and was massively accumulated in heterogeneous vesicle-like structures with bright fluorescence in all the cytoplasm. Therefore the defect in Bgs1 localization to the plasma membrane might be responsible of the defective cell wall in cdc42L160S cells. Additionally, these results unveiled a major difference in the trafficking mechanisms of Bgs1 and Bgs4. It is possible that, as has been described for S. cerevisiae chitin synthases Chs3 and Chs2,Citation28 Bgs1 and Bgs4 trafficking diverges after endocytosis with Bgs1, as Chs2, being delivered to the vacuole and Bgs4, instead, being mainly recycled to the plasma membrane. In this hypothetical situation, Cdc42 would mainly regulate the transport to the vacuole, and not the recycling to the plasma membrane.
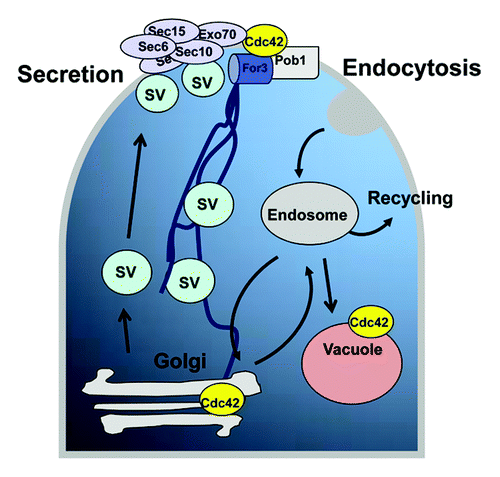
In summary, as presented in , Cdc42 emerges as a key factor that regulates major processes such as the actin-directed vesicle delivery, exocyst function and endosome to vacuole fusion in an independent but coordinated way that leads to polarized growth. The analysis of the inputs that control Cdc42 for the correct coupling of these processes will be necessary to understand how polarized growth takes place.
Figure 2. Cdc42 and trafficking pathways in fission yeast. Proteins can be secreted or targeted to the cell surface in secretory vesicles (SV) that are directed via actin cables and are tethered by the exocyst protein complex to specific sites of the plasma membrane. Molecules taken up from the plasma membrane are delivered to early endosomes by an endocytic process that might lead to a route of recycling or to complete degradation in the vacuole. Cdc42 regulates the actin-directed vesicle delivery to the plasma membrane, the exocyst function, and the endosome to vacuole fusion.
Acknowledgments
We thank Felice Kelly for advice and technical help. This work was supported by Grant BFU2010–15641 from the Comisión Interministerial de Ciencia y Tecnología, Spain. We acknowledge the institutional support granted to the IBFG by the Ramón Areces Foundation during 2011–2012. M. Estravís is receptor of a Titulado Superior contract founded by Junta de Castilla y León and Fondo Social Europeo.
References
- He B, Guo W. The exocyst complex in polarized exocytosis. Curr Opin Cell Biol 2009; 21:537 - 42; http://dx.doi.org/10.1016/j.ceb.2009.04.007; PMID: 19473826
- Martin SG, Rincón SA, Basu R, Pérez P, Chang F. Regulation of the formin for3p by cdc42p and bud6p. Mol Biol Cell 2007; 18:4155 - 67; http://dx.doi.org/10.1091/mbc.E07-02-0094; PMID: 17699595
- Rincón SA, Ye Y, Villar-Tajadura MA, Santos B, Martin SG, Pérez P. Pob1 participates in the Cdc42 regulation of fission yeast actin cytoskeleton. Mol Biol Cell 2009; 20:4390 - 9; http://dx.doi.org/10.1091/mbc.E09-03-0207; PMID: 19710424
- Bendezú FO, Martin SG. Actin cables and the exocyst form two independent morphogenesis pathways in the fission yeast. Mol Biol Cell 2011; 22:44 - 53; http://dx.doi.org/10.1091/mbc.E10-08-0720; PMID: 21148300
- Estravís M, Rincón SA, Santos B, Pérez P. Cdc42 regulates multiple membrane traffic events in fission yeast. Traffic 2011; 12:1744 - 58; http://dx.doi.org/10.1111/j.1600-0854.2011.01275.x; PMID: 21899677
- Irazoqui JE, Gladfelter AS, Lew DJ. Scaffold-mediated symmetry breaking by Cdc42p. Nat Cell Biol 2003; 5:1062 - 70; http://dx.doi.org/10.1038/ncb1068; PMID: 14625559
- Fairn GD, Hermansson M, Somerharju P, Grinstein S. Phosphatidylserine is polarized and required for proper Cdc42 localization and for development of cell polarity. Nat Cell Biol 2011; 13:1424 - 30; http://dx.doi.org/10.1038/ncb2351; PMID: 21964439
- Nakano K, Toya M, Yoneda A, Asami Y, Yamashita A, Kamasawa N, et al. Pob1 ensures cylindrical cell shape by coupling two distinct rho signaling events during secretory vesicle targeting. Traffic 2011; 12:726 - 39; http://dx.doi.org/10.1111/j.1600-0854.2011.01190.x; PMID: 21401840
- Wu H, Rossi G, Brennwald P. The ghost in the machine: small GTPases as spatial regulators of exocytosis. Trends Cell Biol 2008; 18:397 - 404; http://dx.doi.org/10.1016/j.tcb.2008.06.007; PMID: 18706813
- Adamo JE, Rossi G, Brennwald P. The Rho GTPase Rho3 has a direct role in exocytosis that is distinct from its role in actin polarity. Mol Biol Cell 1999; 10:4121 - 33; PMID: 10588647
- Wu H, Turner C, Gardner J, Temple B, Brennwald P. The Exo70 subunit of the exocyst is an effector for both Cdc42 and Rho3 function in polarized exocytosis. Mol Biol Cell 2010; 21:430 - 42; http://dx.doi.org/10.1091/mbc.E09-06-0501; PMID: 19955214
- Roumanie O, Wu H, Molk JN, Rossi G, Bloom K, Brennwald P. Rho GTPase regulation of exocytosis in yeast is independent of GTP hydrolysis and polarization of the exocyst complex. J Cell Biol 2005; 170:583 - 94; http://dx.doi.org/10.1083/jcb.200504108; PMID: 16103227
- Wang H, Tang X, Balasubramanian MK. Rho3p regulates cell separation by modulating exocyst function in Schizosaccharomyces pombe. Genetics 2003; 164:1323 - 31; PMID: 12930742
- Adamo JE, Moskow JJ, Gladfelter AS, Viterbo D, Lew DJ, Brennwald PJ. Yeast Cdc42 functions at a late step in exocytosis, specifically during polarized growth of the emerging bud. J Cell Biol 2001; 155:581 - 92; http://dx.doi.org/10.1083/jcb.200106065; PMID: 11706050
- Coll PM, Trillo Y, Ametzazurra A, Perez P. Gef1p, a new guanine nucleotide exchange factor for Cdc42p, regulates polarity in Schizosaccharomyces pombe. Mol Biol Cell 2003; 14:313 - 23; http://dx.doi.org/10.1091/mbc.E02-07-0400; PMID: 12529446
- Hirota K, Tanaka K, Ohta K, Yamamoto M. Gef1p and Scd1p, the Two GDP-GTP exchange factors for Cdc42p, form a ring structure that shrinks during cytokinesis in Schizosaccharomyces pombe. Mol Biol Cell 2003; 14:3617 - 27; http://dx.doi.org/10.1091/mbc.E02-10-0665; PMID: 12972551
- Kelly FD, Nurse P. Spatial control of Cdc42 activation determines cell width in fission yeast. Mol Biol Cell 2011; 22:3801 - 11; http://dx.doi.org/10.1091/mbc.E11-01-0057; PMID: 21849474
- Edamatsu M, Toyoshima YY. Fission yeast synaptobrevin is involved in cytokinesis and cell elongation. Biochem Biophys Res Commun 2003; 301:641 - 5; http://dx.doi.org/10.1016/S0006-291X(03)00017-2; PMID: 12565827
- Nakamura T, Nakamura-Kubo M, Hirata A, Shimoda C. The Schizosaccharomyces pombe spo3+ gene is required for assembly of the forespore membrane and genetically interacts with psy1(+)-encoding syntaxin-like protein. Mol Biol Cell 2001; 12:3955 - 72; PMID: 11739793
- Kita A, Li C, Yu Y, Umeda N, Doi A, Yasuda M, et al. Role of the Small GTPase Rho3 in Golgi/Endosome trafficking through functional interaction with adaptin in Fission Yeast. PLoS One 2011; 6:e16842; http://dx.doi.org/10.1371/journal.pone.0016842; PMID: 21304827
- Jones L, Tedrick K, Baier A, Logan MR, Eitzen G. Cdc42p is activated during vacuole membrane fusion in a sterol-dependent subreaction of priming. J Biol Chem 2010; 285:4298 - 306; http://dx.doi.org/10.1074/jbc.M109.074609; PMID: 20007700
- Eitzen G, Wang L, Thorngren N, Wickner W. Remodeling of organelle-bound actin is required for yeast vacuole fusion. J Cell Biol 2002; 158:669 - 79; http://dx.doi.org/10.1083/jcb.200204089; PMID: 12177043
- Isgandarova S, Jones L, Forsberg D, Loncar A, Dawson J, Tedrick K, et al. Stimulation of actin polymerization by vacuoles via Cdc42p-dependent signaling. J Biol Chem 2007; 282:30466 - 75; http://dx.doi.org/10.1074/jbc.M704117200; PMID: 17726018
- Aghamohammadzadeh S, Ayscough KR. Differential requirements for actin during yeast and mammalian endocytosis. Nat Cell Biol 2009; 11:1039 - 42; http://dx.doi.org/10.1038/ncb1918; PMID: 19597484
- Cortés JC, Ishiguro J, Durán A, Ribas JC. Localization of the (1,3)beta-D-glucan synthase catalytic subunit homologue Bgs1p/Cps1p from fission yeast suggests that it is involved in septation, polarized growth, mating, spore wall formation and spore germination. J Cell Sci 2002; 115:4081 - 96; http://dx.doi.org/10.1242/jcs.00085; PMID: 12356913
- Cortés JC, Konomi M, Martins IM, Muñoz J, Moreno MB, Osumi M, et al. The (1,3)beta-D-glucan synthase subunit Bgs1p is responsible for the fission yeast primary septum formation. Mol Microbiol 2007; 65:201 - 17; http://dx.doi.org/10.1111/j.1365-2958.2007.05784.x; PMID: 17581129
- Cortés JC, Carnero E, Ishiguro J, Sánchez Y, Durán A, Ribas JC. The novel fission yeast (1,3)beta-D-glucan synthase catalytic subunit Bgs4p is essential during both cytokinesis and polarized growth. J Cell Sci 2005; 118:157 - 74; http://dx.doi.org/10.1242/jcs.01585; PMID: 15615781
- Chuang JS, Schekman RW. Differential trafficking and timed localization of two chitin synthase proteins, Chs2p and Chs3p. J Cell Biol 1996; 135:597 - 610; http://dx.doi.org/10.1083/jcb.135.3.597; PMID: 8909536