Abstract
Notch signaling is an evolutionarily conserved mechanism that defines a key cell fate control mechanism in metazoans. Notch signaling relies on the surface interaction between the Notch receptor and membrane bound ligands in an apposing cell. In our recent study,Citation22 we uncover a non-canonical receptor activation path that relies on a ligand-independent, intracellular activation of the receptor as it travels through the endosomal compartments. We found that Notch receptor, targeted for degradation lysosomal degradation through multivesicular bodies (MVBs) is “diverted” toward activation upon mono-ubiquitination through a synergy between the ubiquitin ligase Deltex, the non-visual β-arrestin Kurtz and the ESCRT-III component Shrub. This activation path is not universal but appears to depend on the cellular context.
Regulation of Notch trafficking
The Notch pathway acts throughout development to link fate choices of a cell to those of its immediate cellular neighbors, ultimately affecting proliferation, apoptosis and differentiation.Citation1-Citation3 Notch malfunction has been associated with aberrant development in all metazoans and with various human diseases.Citation4 The dosage of the Notch signal defines an extraordinarily sensitive parameter that regulates the developmental outcome of signaling. Hence, cellular mechanisms controlling the dosage of activated Notch receptors are of importance for the biology and pathobiology of the pathway.
Numerous studies, mostly in flies, implicated intracellular trafficking of Notch receptor in both ligand-dependent and ligand-independent Notch signaling.Citation5-Citation13 Endocytosis of Notch may result in either down- or upregulation of signaling depending on how the receptor is sorted within the cell.Citation14,Citation15 Although these studies indicated an important role for intracellular trafficking in Notch signaling, the compartments and molecular mechanisms underlying Notch activation—once the receptor enters the endocytic path—remain unclear. Available evidence suggests that there may be several mechanisms that can lead to intracellular Notch activation, either in a ligand-dependent or a ligand-independent manner. The process capable of modulating such intracellular Notch signaling remains unknown, but ligand-independent activation of the receptor has been recently shown to be essential for the normal development of Drosophila blood cells.Citation16
An important element of the endosomal sorting machinery is defined by the ESCRT (endosomal sorting complex required for transport) system.Citation17 ESCRT is crucial in mediating the various steps leading to the sorting of membrane proteins into multivesicular bodies (MVBs) on their way to lysosomal degradation. It is also required for the morphogenesis of intraluminal vesicles and for the sorting of ubiquitinated cargo into these vesicles.Citation17 As elaborated below, we identified an ESCRT member as a Notch signal modifier. The ESCRT system consists of ESCRT-0, -I, -II, -III, and Vps4.Citation17 ESCRT-0, -I, -II contain ubiquitin-binding domains, and are primarily involved in cargo sorting and in the recruitment and activation of ESCRT-III.Citation17 The ESCRT-III subunits Vps20, Vps32 (Shrub), Vps24, Vps4 are assembled in this order, and contribute to the budding and scission of intraluminal vesicles into the vesicles.Citation17,Citation18 Vps4 is the ATPase required for ESCRT-III disassembly.Citation17 Loss of ESCRT function modulates Notch trafficking and leads to ectopic activation of Notch signaling.Citation8-Citation12,Citation19,Citation20 The mechanisms underlying these events are complex, as different members of the ESCRT system exhibit distinct, non-overlapping phenotypic characteristics. For instance, the Vps22, Vps25, Vps36 members of ESCRT-II display non-identical mutant phenotypes involving Notch activity, proliferation, or apoptotic resistance.Citation20 Moreover, there may be tissue specific functions of each component of ESCRTs.Citation20
Shrub is a key modulator of the Notch signal mediated by Deltex/Kurtz
We previously reported interactions between the non-visual β-arrestin Kurtz and the ubiquitin ligase Deltex leading to the regulation of Notch trafficking, its degradation and consequential loss of signaling.Citation21 We identified Shrub, a member of the ESCRT-III complex, as a major modulator of Deltex/Kurtz on Notch signaling.Citation22 Our genetic and molecular studies indicate that absence of Shrub suppresses the classic loss-of-Notch wing-nicking phenotype associated with Notch degradation due to the co-expression of Deltex and Kurtz.Citation22 Use of Cut, a direct downstream target of Notch signals, as a readout of Notch activation revealed that its activation by Deltex can be suppressed by coexpression of Shrub. Expression of Shrub also amplifies the inhibiting effect of Kurtz on Notch.Citation22 But when Shrub activity is inhibited in a Deltex overexpressing background, we found a striking concomitant Notch activation.Citation22 This activation does not depend on the presence of the ligands for Delta or Serrate.Citation22 In addition, biochemical analyses demonstrated that this activation was coupled with an accumulation of mono-ubiquitinated Notch.Citation22 ESCRT-III, through Shrub, seems to play a distinct role in these phenomena, as ESCRT-I and -II components are not required for this Deltex-dependent Notch activation.Citation22 Consistent with this, mutants of ESCRT-I or ESCRT-II did not suppress the wing phenotype caused by the degradation of Notch associated with the overexpression of Deltex and Kurtz.Citation22 Therefore, our data suggest a check-point by ESCRT-III and not ESCRT-I or -II in the ligand-independent and Deltex/Kurtz-dependent regulation of Notch signaling.
Context specific regulation of the Notch signal
A characteristic of the unconventional non-canonical activation of Notch signaling we uncovered, is that it is context dependent. In the wing disc for example, the Notch signal induced by Deltex overexpression is seen mostly in the ventral region.Citation6,Citation22 Similarly, we found that the Notch signal regulated by deltex-shrub is only manifested in the ventral region of the wing disc, indicating that this ligand-independent, intracellular activation of Notch, depends on the cellular context.Citation22 This context specificity is not associated with the glycosyltransferase Fringe (unpublished data), previously shown to regulate the differential activity of the Notch receptor in the ventral vs. dorsal region of the wing disc.Citation23 However, treatment with the lysosomal inhibitor chloroquine abolishes this context specificity, suggesting that this effect depends on factors that regulate chloroquine sensitive lysosomal degradation.Citation22 The mechanism underlying the involvement of lysosomal degradation in this context dependent response is unclear but certainly noteworthy and the subject of future inquiries.
A model for the intracellular activation of Notch
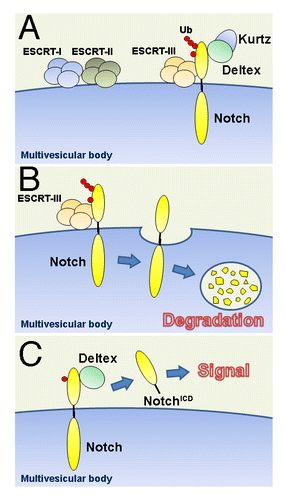
On the basis of these observations, we propose a model for Shrub-Deltex-Kurtz dependent modulation of Notch activation (). Shrub, Deltex, and Kurtz regulate the trafficking of Notch, and modulate the degradation of Notch in the late-endosome/MVBs compartment. In the presence of Shrub, Notch is sorted to the degradation pathway, resulting in downregulating the Notch signal. In the absence of Shrub and/or Kurtz, Deltex promotes mono-ubiquitination, leading to Notch activation
Figure 1. Shrub-Deltex-Kurtz dependent modulation of Notch signaling. (A) The ubiquitination state of the Notch receptor regulates its activation fate as it enters the endocytic path. While some steps in this path have been characterized, others simply illustrate our working hypothesis. (B) Our studies indicate that Deltex in synergy with Kurtz promotes the poly-ubiquitinated state of the receptor. This leads to the degradation of Notch through the MVBs—a step regulated by Shrub that is a core component of the ESCRT-III complex. Our evidence is consistent with the notion that Shrub “surrounds” the ubiquitynated receptor—a role compatible with the previously suggested role of the yeast homolog Snf7. (C) The expression of Deltex, which physically interacts with Notch, favors a mono-ubiquitinated state of the receptor and leads to a ligand-independent activation intracellular activation of Notch (NotchICD: the cleaved, activated form of Notch).
Previous studies have indicated that there may be more than one mechanism that can potentially activate Notch during its endosomal sorting.Citation5-Citation13 Our contribution is based on linking Notch activation to its ubiquitination state, modulated by the synergy of Deltex, Kurtz and Shrub. What remains unclear is the contribution of such a ligand-independent, context-dependent, intracellular activation in the normal biology and in the pathobiology of the Notch signaling pathway.
| Abbreviations: | ||
| ESCRT | = | endosomal sorting complex required for transport |
| MVBs | = | multivesicular bodies |
Acknowledgments
This work was supported by NIH grants NS26084 and CA98402 (S.A-T.), grant 07572 (T.K.), a JSPS Postdoctoral Fellowship for Research Abroad (K.H.) and a Postdoctoral Fellowship from the FSMA (A.S.). We would like to thank K. G. Guruharsha for critically reading the manuscript.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science 1999; 284:770 - 6; http://dx.doi.org/10.1126/science.284.5415.770; PMID: 10221902
- Schweisguth F. Regulation of notch signaling activity. Curr Biol 2004; 14:R129 - 38; PMID: 14986688
- Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol 2006; 7:678 - 89; http://dx.doi.org/10.1038/nrm2009; PMID: 16921404
- Louvi A, Artavanis-Tsakonas S. Notch and disease: A growing field. Semin Cell Dev Biol 2012; In press http://dx.doi.org/10.1016/j.semcdb.2012.02.005; PMID: 22373641
- Coumailleau F, Fürthauer M, Knoblich JA, González-Gaitán M. Directional Delta and Notch trafficking in Sara endosomes during asymmetric cell division. Nature 2009; 458:1051 - 5; http://dx.doi.org/10.1038/nature07854; PMID: 19295516
- Hori K, Fostier M, Ito M, Fuwa TJ, Go MJ, Okano H, et al. Drosophila deltex mediates suppressor of Hairless-independent and late-endosomal activation of Notch signaling. Development 2004; 131:5527 - 37; http://dx.doi.org/10.1242/dev.01448; PMID: 15496440
- Wilkin MB, Carbery AM, Fostier M, Aslam H, Mazaleyrat SL, Higgs J, et al. Regulation of notch endosomal sorting and signaling by Drosophila Nedd4 family proteins. Curr Biol 2004; 14:2237 - 44; http://dx.doi.org/10.1016/j.cub.2004.11.030; PMID: 15620650
- Vaccari T, Bilder D. The Drosophila tumor suppressor vps25 prevents nonautonomous overproliferation by regulating notch trafficking. Dev Cell 2005; 9:687 - 98; http://dx.doi.org/10.1016/j.devcel.2005.09.019; PMID: 16256743
- Thompson BJ, Mathieu J, Sung HH, Loeser E, Rørth P, Cohen SM. Tumor suppressor properties of the ESCRT-II complex component Vps25 in Drosophila. Dev Cell 2005; 9:711 - 20; http://dx.doi.org/10.1016/j.devcel.2005.09.020; PMID: 16256745
- Childress JL, Acar M, Tao C, Halder G. Lethal giant discs, a novel C2-domain protein, restricts notch activation during endocytosis. Curr Biol 2006; 16:2228 - 33; http://dx.doi.org/10.1016/j.cub.2006.09.031; PMID: 17088062
- Vaccari T, Lu H, Kanwar R, Fortini ME, Bilder D. Endosomal entry regulates Notch receptor activation in Drosophila melanogaster. J Cell Biol 2008; 180:755 - 62; http://dx.doi.org/10.1083/jcb.200708127; PMID: 18299346
- Vaccari T, Rusten TE, Menut L, Nezis IP, Brech A, Stenmark H, et al. Comparative analysis of ESCRT-I, ESCRT-II and ESCRT-III function in Drosophila by efficient isolation of ESCRT mutants. J Cell Sci 2009; 122:2413 - 23; http://dx.doi.org/10.1242/jcs.046391; PMID: 19571114
- Wilkin M, Tongngok P, Gensch N, Clemence S, Motoki M, Yamada K, et al. Drosophila HOPS and AP-3 complex genes are required for a Deltex-regulated activation of notch in the endosomal trafficking pathway. Dev Cell 2008; 15:762 - 72; http://dx.doi.org/10.1016/j.devcel.2008.09.002; PMID: 19000840
- Fortini ME. Notch signaling: the core pathway and its posttranslational regulation. Dev Cell 2009; 16:633 - 47; http://dx.doi.org/10.1016/j.devcel.2009.03.010; PMID: 19460341
- Yamamoto S, Charng WL, Bellen HJ. Endocytosis and intracellular trafficking of Notch and its ligands. Curr Top Dev Biol 2010; 92:165 - 200; http://dx.doi.org/10.1016/S0070-2153(10)92005-X; PMID: 20816395
- Mukherjee T, Kim WS, Mandal L, Banerjee U. Interaction between Notch and Hif-alpha in development and survival of Drosophila blood cells. Science 2011; 332:1210 - 3; http://dx.doi.org/10.1126/science.1199643; PMID: 21636775
- Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev Cell 2011; 21:77 - 91; http://dx.doi.org/10.1016/j.devcel.2011.05.015; PMID: 21763610
- Wollert T, Wunder C, Lippincott-Schwartz J, Hurley JH. Membrane scission by the ESCRT-III complex. Nature 2009; 458:172 - 7; http://dx.doi.org/10.1038/nature07836; PMID: 19234443
- Moberg KH, Schelble S, Burdick SK, Hariharan IK. Mutations in erupted, the Drosophila ortholog of mammalian tumor susceptibility gene 101, elicit non-cell-autonomous overgrowth. Dev Cell 2005; 9:699 - 710; http://dx.doi.org/10.1016/j.devcel.2005.09.018; PMID: 16256744
- Herz HM, Woodfield SE, Chen Z, Bolduc C, Bergmann A. Common and distinct genetic properties of ESCRT-II components in Drosophila. PLoS One 2009; 4:e4165; http://dx.doi.org/10.1371/journal.pone.0004165; PMID: 19132102
- Mukherjee A, Veraksa A, Bauer A, Rosse C, Camonis J, Artavanis-Tsakonas S. Regulation of Notch signalling by non-visual β-arrestin. Nat Cell Biol 2005; 7:1191 - 201; http://dx.doi.org/10.1038/ncb1327; PMID: 16284625
- Hori K, Sen A, Kirchhausen T, Artavanis-Tsakonas S. Synergy between the ESCRT-III complex and Deltex defines a ligand-independent Notch signal. J Cell Biol 2011; 195:1005 - 15; http://dx.doi.org/10.1083/jcb.201104146; PMID: 22162134
- Fleming RJ, Gu Y, Hukriede NA. Serrate-mediated activation of Notch is specifically blocked by the product of the gene fringe in the dorsal compartment of the Drosophila wing imaginal disc. Development 1997; 124:2973 - 81; PMID: 9247339