Abstract
Here, we focus on synthetic lethal genetic interactions, examples of genetic enhancements, where mutations in two different genes result in lethality but only when present together. We recently identified the synthetic lethal network around the PKC1 gene encoding the essential protein kinase C of yeast. We found that this network is heavily enriched for interactions with genes whose products are closely linked to Pkc1 signallng in vivo. Here, we show that: the PKC1 gene elicits a distinct spectrum of genetic interactions to SLT2, encoding a non-essential component of the very same signaling pathway. We also show that the terminal phenotype underlying the synthetic lethal network around PKC1 is not uniform. Synthetic lethal genetic networks thus appear to be very heterogeneous in nature with important implications for what functional relationships can be discovered from them.
Pair wise synthetic genetic interactions, otherwise known as phenotype enhancements, occur when mutations in two different genes cause a strong phenotypic effect but only when present together. Synthetic lethality occurs where two mutations together result in inviability.Citation1 Synthetic lethal interactions can now be systematically uncovered for any given “query” gene of interest in yeast.Citation2–Citation4
What functional relationships between gene products underlie such synthetic lethal interactions between genes? Clearly, each interacting pair of gene products must buffer the cell from the full consequences of the absence of the other. For synthetic lethal interactions between pairs of null (complete loss-of-function) alleles in non-essential genes, only mutations disabling independent buffering functions could synergiseCitation1 since each null mutation alone completely inactivates the local “pathway” in which the gene product acts. Functional relationships underlying synthetic interactions between null alleles of non-essential genes are always parallel and can be remote.
In contrast, partly functional (hypomorphic) mutations in essential genes are thought to sensitize the organism to mutations that affect the same, rather than a parallel, pathway or process.Citation1,Citation5–Citation8 Unfortunately, hypomorphic-hypomorphic interactions between pairs of essential genes are difficult to identify systematically, although ad hoc observations support a high frequency of “within pathway” relationhips.Citation9 However, hypomorphic-null interactions involving an essential and a non-essential genes can be readily and systematically uncovered and should also be enriched for “within pathway” interactions, at least to some as yet unknown extent. For example, a hypomorphic mutation lowering activity of an essential hub protein in a signaling pathway may sensitize cells to null mutations that partly disable the same pathway, e.g., by fully disabling a non-essential branch feeding into or out of that essential hub.
We recently determined and analyzed the hypomorphic-null synthetic lethal network around the essential yeast PKC1 gene.Citation10 Pkc1, the only yeast isozyme of protein kinase C, functions as a key hub in the highly branched “Cell Wall Integrity” (CWI) signaling pathway that primarily acts to prevent cell lysis (reviewed in ref. Citation11). We found the absence of any one of 21 non-essential genes renders cells hypersensitive to lowered Pkc1 activity.Citation10 Analysis of the interactions indicated that “within pathway” interactions dominate this PKC1 network. Nine of the genes (43%) encode known or novel components of the CWI pathway (). Furthermore, a chemical-genetic epitasis analysis indicates that two thirds of PKC1 interactions are “within pathway”.Citation10
Why has this enrichment not been noticed in other essential gene networks? First, large scale networks have tended to be mixed, but are dominated by null-null interactions between non-essential gene pairs, interactions that should be parallel.Citation2–Citation4 Little attention has been paid to the behavior of individual constituent subtypes of interactions. Second, functional relationships in large-scale networks have been estimated by indirect and limited in silico analyses.Citation2–Citation4 Third, it is always possible that PKC1 and genes involved in the CWI pathway behave abnormally and are not representative of network behavior. Here, we address this latter possibility.
Is enrichment for “within pathway” interactions a property of any network involving CWI pathway components? The null-null synthetic lethal network around SLT2, lacking a key non-essential downstream component of the CWI pathway (), has been determined both by systematic and non-systematic analyses.Citation2,Citation12 The PKC1 and SLT2 networks overlap only slightly, sharing four target genes, JNM1, SWI4, BNI1 and SPA2, representing 19% and 5% of the two networks respectively (). This lack of overlap is not due to a high rate of false positives since each network is confirmed experimentally. Nor is it due to a high rate of false negatives since we have directly and experimentally tested the PKC1 network for null-null interactions with SLT2 and find very few (Krause SA and Gray JV, unpublished work). This lack of overlap is robust. High “within pathway” content is not an inherent property of CWI-related networks. Rather, we conclude that null-null and hypomorphic-null networks behave very differently to each other, even for components of the CWI pathway.
Our data are in apparent conflict with the results of recent work exploiting titration-expression alleles of essential genes in which there was no evidence for enrichment of “within pathway” characteristics in titration-null and titration-titration interactions.Citation13 It is possible that the PKC1 network is unusual. Alternatively, it may be that titration expression alleles are unusual, and not truly hypomorphic. Indeed, these alleles are unusually profligate in eliciting synthetic lethal interactions, much more so that are hypomorphic alleles of PKC1 or of other essential genes examined to date.Citation3,Citation13 It should be noted that the behavior of titration expression alleles is based on limited data and may be in error. The jury is out but it seems likely that the nature of the interacting alleles (null, hypomorphic, titration expression, etc.), rather than the nature of the genes (essential or not; CWI pathway or not), is the dominant feature in determining the functional relationship underlying a synthetic genetic interaction.
Most genes, irrespective of allele type, are still surprisingly promiscuous in entering into synthetic lethal genetic interactions. Why? The nature of the phenotype being examined may be key. Some phenotypes are very defined and specific, others are not. In the case of synthetic lethality, there are many different possible ways for a double mutant cell to appear inviable: they could suffer apoptotic death, a necrotic death (e.g., cell lysis), or arrest in some viable state that is incapable of active proliferation. A synthetic lethal network around a given gene may thus be comprised of many sub-networks, each with a specific limiting terminal phenotype.
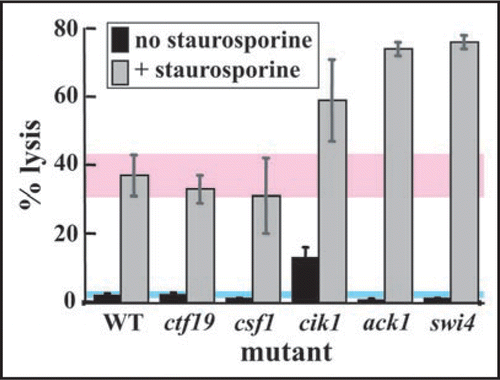
We examined a subset of the PKC1 synthetic lethal network. We assessed the terminal phenotype of null mutants at a concentration of the Pkc1 inhibitor staurosporine that selectively prevents growth of the mutant but not wild-type cells. We quantified the extent of cell lysis, the classic CWI pathway mutant phenotype.Citation14 As shown in , we found that some null mutations exacerbate the extent of cell lysis caused by staurosporine, but others do not. Terminal phenotype is thus heterogeneous, with different phenotypes being limiting in different genetic backgrounds. In this case, we might expect Pkc1 to have multiple roles, only some of which are associated with cell integrity. This is indeed true.Citation11
We conclude that different terminal phenotypes can underlie the observed synthetic lethal network around a given gene, partly explaining the high frequency of synthetic lethal genetic interactions that are observed. Analysis of terminal phenotype associated with a synthetic lethal interaction should also provide potentially valuable insights into the different in vivo roles of the interacting gene products.
Figures and Tables
Figure 1 The synthetic genetic interaction network around PKC1 identified components of the CWI pathway and is distinct from the network around SLT2, encoding a non-essential component of the pathway. (A) Simplified schematic of the CWI pathway indicating where the products of nine of the 21 genes that are synthetic lethal with PKC1 lie in the pathway. Known or newly confirmed components are in yellow. Transcriptional targets are shown in orange. Protein protein interactions are indicated by blue lines. Filled arrows indicate direct activations: dashed arrows indicate indirect activations. (B) Schematic indicating the extent of overlap between the hypomorphic-null synthetic lethal network around PKC1 and the null-null network around the non-essential SLT2, encoding a key downstream component of the pathway (A). The network around SLT2 is derived predominantly from data from systematic screens.Citation3 Yellow and orange target genes are as for (A) above. Purple target gene products are implicated as acting in the CWI pathway via epistasis analysis alone.Citation10
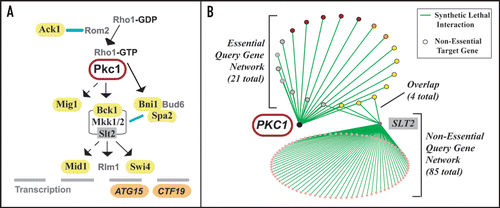
Figure 2 The synthetic lethal network around PKC1 is underpinned by heterogeneity of terminal phenotype. WT and mutant cultures in early logarithmic phase were grown in the absence or presence of staurosporine (30 µg/mL) in rich medium at 25°C for 4 hours. Percent (%) lysis was determined by propidium iodide staining.Citation14 Untreated cells (wild-type and single mutant) showed little lysis. Treatment of wild-type cells with staurosporine caused significant lysis (37%) although growth of the culture overall was not inhibited (data not shown). The extent of cell lysis varied dramatically between mutants in the presence of staurosporine, even though growth of all mutant cell cultures was inhibited by this concentration of the drug (data not shown). The synthetic lethal network around PKC1 is thus associated with heterogeneity of phenotype: some combinations compromise cell integrity, others do not.
Acknowledgements
This research was supported by grants from the Biotechnology and Biological Sciences Research Council, UK (C20144 and BB/E011632 to Joseph V. Gray). We thank Hong Xu and Huiming Ding for discussions. The authors declare that they have no competing financial interests.
Addendum to:
References
- Ooi SL, Pan X, Peyser BD, Ye P, Meluh PB, Yuan DS, Irizarry RA, Bader JS, Spencer FA, Boeke JD. Global synthetic-lethality analysis and yeast functional profiling. Trends Genet 2006; 22:56 - 66
- Tong AH, Evangelisa M, Parsons AB, Xu H, Bader GD, Page N, Robinson M, Raghibizadeh S, Hogue CW, Bussey H, Andrews B, Tyers M, Boone C. Systematic genetic analysis with ordered arrays of yeast mutants. Science 2001; 294:2364 - 2368
- Tong AH, Lesage G, Bader GD, Ding H, Xu H, Xin X, Young J, Berriz GF, Brost RL, Chang M, Chen Y, Cheng X, Chua G, Friesen H, Goldberg DS, Haynes J, Humphries C, He G, Hussein S, Ke L, Krogan N, Li Z, Levinson JN, Lu H, Menard P, Munyana C, Parsons AB, Ryan O, Tonikian R, Roberts T, Sdicu AM, Shapiro J, Sheikh B, Suter B, Wong SL, Zhang LV, Zhu H, Burd CG, Munro S, Sander C, Rine J, Greenblatt J, Peter M, Bretscher A, Bell G, Roth FP, Brown GW, Andrews B, Bussey H, Boone C. Global mapping of the yeast genetic interaction network. Science 2004; 303:808 - 813
- Pan X, Yuan DS, Xiang D, Wang X, Sookhai-Mahadeo S, Bader JS, Heiter P, Spencer F, Boeke JD. A robust toolkit for functional profiling of the yeast genome. Mol Cell 2004; 16:487 - 496
- Guarente L. Synthetic enhancement in gene interaction: a genetic tool come of age. Trends Genet 1993; 9:362 - 366
- Cullen LM, Arndt GM. Genome-wide screening for gene function using RNAi in mammalian cells. Immunol Cell Biol 2005; 83:217 - 223
- Mathey-Prevot B, Perrimon N. Drosophila genome-wide RNAi screens: are they delivering the promise?. Cold Spring Harb Symp Quant Biol 2006; 71:141 - 148
- Sugimoto A. High-throughout RNAi in Caenorhabditis elegans: genome-wide screens and functional genomics. Differentation 2004; 72:81 - 91
- Hartman JL, Garvik B, Hartwell L. Principles for the buffering of genetic variation. Science 2001; 291:1001 - 1004
- Krause SA, Xu H, Gray JV. The synthetic genetic network around PKC1 identifies novel modulators and components of protein kinase C signaling in yeast. Eukaryot Cell 2008; 7:1880 - 1887
- Levin DE. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol Mol Biol Rev 2005; 69:262 - 291
- Reguly T, Breitkreutz A, Boucher L, Breitkreutz BJ, Hon GC, Myers CL, Parsons A, Friesen H, Oughtred R, Tong A, Stark C, Ho Y, Botstein D, Andrews B, Boone C, Troyanskya OG, Ideker T, Dolinski K, Batada NN, Tyers M. Comprehensive curation and analysis of global interaction networks in Saccharomyces cerevisiae. J Biol 2006; 5:11
- Davierwala AP, Haynes J, Li Z, Brost RL, Robinson MD, Yu L, Mnaimneh S, Ding H, Zhu H, Chen Y, Cheng X, Brown GW, Boone C, Andrews BJ, Hughes TR. The synthetic genetic interaction spectrum of essential genes. Nat Genet 2005; 37:1147 - 1152
- Krause SA, Gray JV. The protein kinase C pathway is required for viability in quiescence in Saccharomyces cerevisiae. Curr Biol 2002; 12:588 - 593