Abstract
Neuronal adaptation has been studied extensively in visual motion-sensitive neurons of the fly Calliphora vicina, a model system in which the computational principles of visual motion processing are amenable on a single-cell level. Evidenced by several recent papers, the original idea had to be dismissed that motion adaptation adjusts velocity coding to the current stimulus range by a simple parameter change in the motion detection scheme. In contrast, linear encoding of velocity modulations and total information rates might even go down in the course of adaptation. Thus it seems that rather than improving absolute velocity encoding motion adaptation might bring forward an efficient extraction of those features in the visual input signal that are most relevant for visually guided course control and obstacle avoidance.
Visual motion plays an important role in behavioral control.Citation1 Blowflies are equipped with a set of visual motion-sensitive tangential cells (TCs).Citation2 These neurons have large receptive fields and a high selectivity for visual motion patterns occurring during certain translational or rotational movements.Citation3,Citation4 Visual image flow experienced by a fast flying animal like a blowfly changes dramatically in its intensity and statistical properties depending on the environment and, in particular, the animal's current flight maneuvers.Citation5,Citation6 This may pose a problem to the neuronal machinery, because neuronal input-output functions are inevitably constrained by thresholds and saturation limits. As a consequence, the working range in which a neuron can effectively respond to small changes in input intensity with a high signal-to-noise ratio can be much smaller than the range of inputs that may be encountered.Citation7 It is thus not surprising that in the first accounts on adaptation in fly TCs it had been assumed that motion adaptation leads to an improved encoding of changes in velocity by aligning the neuronal velocity-response function with the range of velocities currently present in the input.Citation8,Citation9
The results of a recent study are hardly compatible with the notion that motion adaptation leads to an improved representation of motion velocity by fly TCs, e.g., by tuning neuronal input-output functions to the current demands.Citation10 In this study TCs were stimulated with a drifting grating, which changed its velocity in a random fashion (). When analyzing how well the time-varying stimuli can be recovered from the neuronal responses by reverse reconstruction it turned out, that adaptation led to a change for the worse rather than an improvement. A change in reverse reconstruction performance can have two reasons. The first one is a decrease in signal-to-noise ratio in the course of adaptation. Surprisingly, this was not the case, although the neuronal response amplitude was nearly halved with adaptation. The second possibility is that adaptation leads to an increase in nonlinear stimulus processing by the neurons. Nonlinear processing would degrade reverse reconstruction, because this is based entirely on linear filtering. Thus it was shown that stimulus encoding by TCs shifts from a fairly linear representation of velocity modulations in the non-adapted state to a more and more nonlinear representation in the adapted state.
Which type of nonlinear process is becoming more and more relevant in visual motion processing by fly TCs in the course of motion adaptation? And what functional benefit results from nonlinear processing—a benefit that may count more than precise tracking of absolute velocity? First of all, the study gives a hint where this adaptation- induced change in coding is located. Two types of TCs were studied, one of which, the VS-neuron, is thought to receive direct input from local motion-detector elements in the periphery. The second type of TC, the V1-neuron, is postsynaptic to the first and delivers motion information to the contralateral brain hemisphere. Adaptation effects were similar in the two cell types, indicating that they are either generated in the presynaptic neuron or even further in the periphery. Second, it was observed that in the adapted state fairly strong neuronal responses were present when the velocity of the grating pattern changed abruptly, whereas the neuronal response during phases of comparatively slow velocity changes was attenuated more. Hints into a similar direction come from another recent study, which addressed the impact of adaptation on information transmission by a fly TC.Citation11 In this study the neuron was adapted to different velocity stimuli and the responses were fit by a correlation-type motion detector model to assess which of the model parameters change with adaptation. It was found that a shortening of the time constant of a high-pass filter in the periphery provides the best explanation for the observed adaptation phenomena. Such a change could have the effect of emphasizing abrupt changes in the input at the expense of slowly varying inputs. Intriguingly, the system's overall information transmission was not optimized by adaptation. In contrast, a model in which the peripheral high-pass filter was held in the non-adapted state reached higher information rates than a fully adaptable model.
The recent findings described above are in some respects reminiscent of the results obtained in the first report on motion adaptation in fly TCs.Citation8 There, a drifting grating was presented that had a constant velocity over a sustained period of time, apart from brief steps to a higher or a lower velocity. The neuronal response to these velocity discontinuities was enhanced with adaptation, although the response to baseline velocity was drastically reduced. Originally, it was suggested that adaptation caused a shift in the velocity-response function. However, two observations render this obvious explanation unlikely and imply more complex adaptation-induced changes. First, shifts in the velocity-response function with adaptation appear not to be present in TCs. This was initially shown for adaptation with constant velocityCitation12 and recently corroborated when using randomly modulated velocity.Citation10 Second, an enhanced sensitivity to stimulus discontinuities can be found also when other stimulus parameters than motion velocity are transiently changed, e.g., pattern contrast.Citation13 What does this mean in a functional context? When the system's overall excitability is reduced with adaptation, but abrupt changes in any of the parameters of the stimulus are still able to elicit strong responses, the system might operate as a “novelty detector”. The idea of improved novelty detections by adaptation has called much attention in the auditory system.Citation14,Citation15 Here it is particularly useful to filter novel stimuli from background noise, e.g., by adaptation that is specific for different frequencies of sound. However, such input-specific adaptation might be more difficult to implement in motion vision than in auditory processing, where different frequencies can be separately processed from early on in the system.
An interesting alternative mechanism to accentuate abrupt changes in an input signal was recently demonstrated by a computational network model of mammalian visual cortex.Citation16 In this model presynaptic spike-frequency adaptation was combined with synaptic short-term depression. The postsynaptic neurons decreased their activity during tonic activation of the network, but they were still able to respond strongly whenever the input current given to the presynaptic neurons was changed abruptly. In the fly visual system, the cellular basis of adaptation is largely unknown. However, similar to the mechanisms underlying spike-frequency adaptation in mammalian visual cortex, an activity-dependent conductance that is activated by sustained excitatory stimulation has been demonstrated in fly TCs.Citation17–Citation19
In how far is the processing of natural visual input affected by an accentuation of stimulus discontinuities in the course of adaptation, or “novelty detection”? In a recent study retinal image sequences as seen during flight were reconstructed from the flight trajectory and replayed during recordings from TCs.Citation20 Repeated presentation of these natural image sequences caused a strong decline in the neuronal response. However, when virtual objects were added to the image sequences, the object-induced responses remained much higher than the responses elicited by pure background motion. This result implies that motion adaptation can enhance the detectability of objects, which elicit a prominent discontinuity in image flow during flight. Studies that address in more detail how the dynamics of motion adaptation interact with the complex spatio-temporal profile of natural visual stimuli may in the future help understand the functional benefits of adaptation under real-life conditions.
Figures and Tables
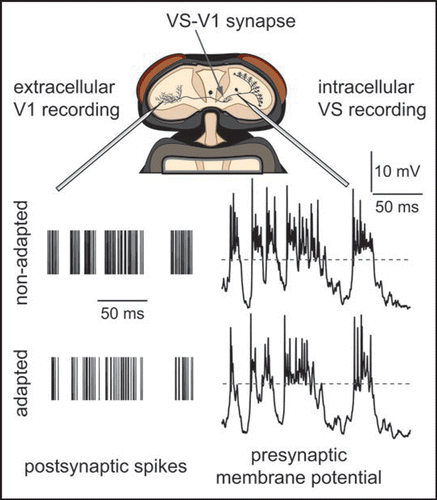
Figure 1 Adaptation of fly visual motion-sensitive neurons. Two synaptically coupled types of neurons were simultaneously recorded in vivo during presentation of a grating pattern. Repeated identical sequences of random velocity modulations of the pattern were presented. Top, schematic of the fly head with the exposed recording site. Bottom, membrane potential responses of the presynaptic VS-neuron (right) and trains of action potentials of the postsynaptic V1-neuron (left). VS-neurons transfer signals at synapses by graded potentials. Dashed horizontal line represents resting membrane potential of VS prior to stimulation. Action potentials of V1 are indicated by vertical lines.
Acknowledgements
Thanks to C. Spalthoff for his contribution to the illustration.
Addendum to:
References
- Egelhaaf M, Kern R. Vision in flying insects. Curr Opin Neurobiol 2002; 12:699 - 706
- Hausen K. Ma A. The lobula-complex of the fly: structure, function and significance in visual behaviour. Photoreception and Vision in Invertebrates 1984; New York Plenum 523 - 555
- Egelhaaf M, Kern R, Krapp HG, Kretzberg J, Kurtz R, Warzecha A-K. Neural encoding of behaviourally relevant motion information in the fly. Trends Neurosci 2002; 25:96 - 102
- Krapp HG. Lappe M. Neuronal matched filters for optic flow processing in flying insects. Neuronal Processing of Optic Flow 2000; San Diego, CA Academic Press 93 - 120
- Van Hateren JH, Kern R, Schwertfeger G, Egelhaaf M. Function and coding in the blowfly H1 neuron during naturalistic optic flow. J Neurosci 2005; 25:4343 - 4352
- Boeddeker N, Lindemann JP, Egelhaaf M, Zeil J. Responses of blowfly motion-sensitive neurons to reconstructed optic flow along outdoor flight paths. J Comp Physiol A 2005; 189:401 - 409
- Laughlin SB. A simple coding procedure enhances a neuron's information capacity. Z Naturforsch 1981; 36:910 - 912
- Maddess T, Laughlin SB. Adaptation of the motion-sensitive neuron H1 is generated locally and governed by contrast frequency. Proc R Soc Lond B 1985; 225:251 - 275
- Shi J, Horridge GA. The H1 neuron measures change in velocity irrespective of contrast frequency, mean velocity or velocity modulation frequency. Proc R Soc Lond B 1991; 331:205 - 211
- Kalb J, Egelhaaf M, Kurtz R. Adaptation of velocity encoding in synaptically coupled neurons in the fly visual system. J Neurosci 2008; 28:9183 - 9193
- Safran MN, Flanagin VL, Borst A, Sompolinsky H. Adaptation and information transmission in fly motion detection. J Neurophysiol 2007; 98:3309 - 3320
- Harris RA, O'Carroll DC, Laughlin SB. Adaptation and the temporal delay filter of fly motion detectors. Vision Res 1999; 39:2603 - 2613
- Kurtz R, Meyer HG, Egelhaaf M, Kern R. Enhanced sensitivity to stimulus discontinuities by adaptation of a fly visual motion-sensitive neuron. Götttingen Neurobiol Conf Abstr 2009;
- Condon CD, Weinberger NM. Habituation produces frequency-specific plasticity of receptive fields in the auditory cortex. Behav Neurosci 1991; 105:416 - 430
- Ulanovsky N, Las L, Nelken I. Processing of low-probability sounds by cortical neurons. Nat Neurosci 2003; 6:391 - 398
- Puccini GD, Sanchez-Vives MV, Compte A. Selective detection of abrupt input changes by integration of spike-frequency adaptation and synaptic depression in a computational network model. J Physiol Paris 2006; 100:1 - 15
- Kurtz R, Dürr V, Egelhaaf M. Dendritic calcium accumulation associated with direction-selective adaptation in visual motion-sensitive neurons in vivo. J Neurophysiol 2000; 84:1914 - 1923
- Harris RA, O'Carroll DC, Laughlin SB. Contrast gain reduction in fly motion adaptation. Neuron 2000; 28:595 - 606
- Kurtz R. Direction-selective adaptation in fly visual motion-sensitive neurons is generated by an intrinsic conductance-based mechanism. Neuroscience 2007; 146:573 - 583
- Liang P, Kern R, Egelhaaf M. Motion adaptation enhances object-induced neural activity in three-dimensional virtual environment. J Neurosci 2008; 28:11328 - 11332