Abstract
The lipids present in the nuclei play different roles in relation to their localization. They are composed by high levels of phosphatidylcholine and sphingomyelin strongly linked with cholesterol. The nuclear lipid composition shows many modifications during cell life due to the presence and activity of some specific enzymes such as sphingomyelinase, sphingomyelin-synthase, reverse sphingomyelin-syntase and phosphatidylcholine-specific phospholipase C. These lipids are associated with a small amount of DNA, with the new-synthesized double-strand RNA, and with proteins to form an intranuclear complex that it is not possible to extract with the techniques used for nuclear membrane and chromatin purification. The intranuclear complex represents a section of inner nuclear membrane that binds to the active chromatin. In a recent paper, we have demonstrated that this complex actually constitutes the lipid microdomains present in the inner nuclear membrane and represents a platform for the transcription process. The possible model of action is reported in this addendum article.
Previous observations have demonstrated that in the nucleus the lipid component is present in various subnuclear compartments playing different roles.Citation1 In nuclear membrane and nuclear matrix, it regulates the fluidity, in chromatin it participates in signal transduction and in intranuclear complex it maintains the double/strand RNA structure influencing its transfer to the cytoplasm.Citation2 During the years the main problem facing this research was that the nuclear lipid could regulate cellular functions. It has been shown that in the nucleus there are energy-independent enzymes that allow lipid metabolism to occur independently from metabolism that occurs in other subcellular structures.Citation3 In particular, the sphingomyelin (SM) is degraded by sphingomyelinase (SMase) and used by reverse-sphingomyelin-synthase (RSM-synthase) to synthesize phosphatidylcholine (PC) using the phosphocholine (PPC) group of SM and free DAG. Both these reactions increase the nuclear ceramide pool, reducing diacylglycerol (DAG)/ceramide ratio.Citation1 Also, PC is degraded by phosphatidylcholine-specific phospholipase C (PC-PLC) and used by sphingomyelin-synthase (SM-synthase) to synthesize SM using PPC group of PC and free ceramide. Both these reactions increase the nuclear DAG pool, increasing DAG/ceramide ratio.Citation1 Recent results clearly highlight that in the nuclear membrane the metabolism of PC is independent from that of SM and vice-versa, whereas, in chromatin, a crosstalk between the two lipid metabolisms exists.Citation3 Moreover there are active chromatin fractions associated with inner nuclear membrane that are impossible to isolate with the techniques used for nuclear membrane or chromatin purification but isolated with specific biochemical techniques and called the “intranuclear complex”.Citation4 This complex contains a small amount of DNA, the new synthesized RNA, STAT3 transcription factor protein and the lipid component constituted only by PC, SM and cholesterol (CHO).Citation4,Citation5 This specific RNA is defined as a double strand RNA (dsRNA) because of its resistance to RNAse treatment.Citation4
Since the treatment of the complex with SMase reduces the SM level freeing PC and CHO and leads the dsRNA into a single strand RNA, it has been supposed that these lipids interact to protect the newly synthesized RNA. We have recently demonstrated that these lipids are organized to form intranuclear lipid microdomains.Citation6 The protein marker for these microdomains is STAT3 and they correspond in composition to the intranuclear complex. Moreover, some proteins of these intranuclear microdomains are represented by N-SMase, SM-synthase and RSM-synthase. The equilibrium between the activity of these enzyme is responsible for the exact ratio of PC: SM: CHO that is characteristic of the lipid microdomains.Citation6 Therefore the intranuclear microdomains are present in the inner nuclear membrane and represent a specific section able to bind the active chromatin. During cell proliferation, nuclear microdomains are characterized by an increased SM content due to higher activity of SM-synthase, which increases the rigidity of the structure facilitating the transcription process.Citation6
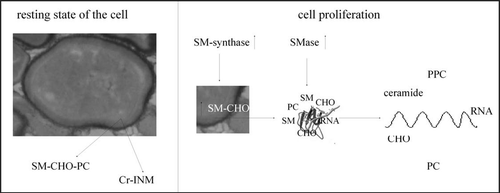
The possible model of the molecular mechanisms that occur in the intranuclear lipid microdomains is described in : (i) in the rest state of the cells, the inner nuclear membrane contains the lipid microdomains characterized by an equilibrium between the activities of N-SMase, SM-synthase and RSM-synthase with the consequent ratio PC: SM: CHO of 1:1:1; these microdomains link chromatin fractions. (ii) during cell proliferation the nuclear lipid microdomains are more rigid because SM synthesis is stimulated; the microdomains become a platform for the transcription process. (iii) the RNA is synthesized and the lipid microdomains protect it, favoring the acquisition of a double-strand and/or a loop conformation. (iv) the activation of SMase degrades SM freeing PC and CHO; the structure of lipid microdomains are modified and the RNA becomes mono-strand and transfers into the cytoplasm.
Abbreviations
| CHO | = | cholesterol |
| DAG | = | diacylglycerol |
| dsRNA | = | double-strand RNA |
| PC | = | phosphatidylcholine |
| PC-PLC | = | phosphatidylcholine-specific phospholipase C |
| PPC | = | phosphocholine |
| RSM-synthase | = | reverse sphingomyelin-synthase |
| SM | = | sphingomyelin |
| SMase | = | sphingomyelinase |
| SM-synthase | = | sphingomyelin-synthase |
Figures and Tables
Acknowledgements
We wish to acknowledge support from Ministero dell' Università e Ricerca (PRIN project), ASI (Agenzia Spaziale Italiana), and the Fondazione Cassa di Risparmio di Perugia. We also thank Remo Lazzarini for the technical assistance, and Ryan Rhome for the manuscript revision.
Addendum to:
References
- Albi E, Viola Magni MP. The role of intranuclear lipids. Biol Cell 2004; 96:657 - 667
- Albi E, Viola Magni MP. Sphingomyelin: a small-big molecule in the nucleus. Rec Res Dev Biophys Biochem 2006; 661:211 - 227
- Albi E, Lazzarini R, Viola Magni M. Phosphatidylcholine/sphingomyelin metabolism crosstalk inside the nucleus. Biochem J 2008; 410:381 - 389
- Rossi G, Magni MV, Albi E. Sphingomyelin-cholesterol and double stranded RNA relationship in the intranuclear complex. Arch Biochem Biophys 2007; 459:27 - 32
- Rossi G, Viola Magni M, Albi E. Signal transducer and activator of transcription 3 and sphingomyelin metabolism in intranuclear complex during cell proliferation. Arch Biochem Biophys 2007; 464:138 - 143
- Cascianelli G, Villani M, Tosti M, Marini F, Barroccini E, Viola Magni M, Albi E. Lipid microdomains in cell nucleus. Mol Biol Cell 2008; In press [Epub ahead of print]