Abstract
CaMKII, calcium/calmodulin dependent protein kinase, is an active kinase in the cell that phosphorylates a number of substrates including several cytoskeletal and signaling proteins. In addition to kinase activity, the β isoform of CaMKII also contains an F-actin binding region. We recently identified a new F-actin rich structure in developing cortical neurons that endogenous CaMKIIβ bound. In nonneuronal cells and dendrite spines of hippocampal neurons where an interaction between CaMKIIβ and F-actin has been identified, CaMKIIβ was involved in regulating the differentiation of dendrite spines and formation of synapses. In this study, we took advantage of the temporal and spatial regulation of CaMKII isoforms to reveal a specific role for CaMKIIβ in binding and stability of a novel F-actin rich structure. We used FRAP and colocalization assays in this CaMKIIβ rich system to demonstrate a structural, rather than enzymatic, role of CaMKIIβ. In this addendum, we further discuss the significance of this study and the possible implication to the field.
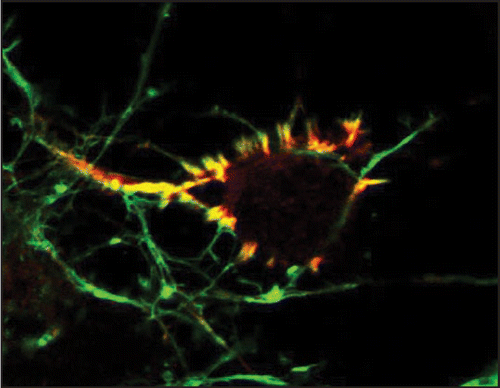
CaMKII is an evolutionarily conserved multimeric protein encoded by four isoforms in higher vertebrates: α, β, γ and δ.Citation1 CaMKII is an abundant protein and constitutes up to 1∼2% of total protein in the adult brain.Citation2,Citation3 Although all isoforms of CaMKII can be found in the brain, CaMKIIα and β predominate and preferentially form α and β holoenzymes and αβ heteroenzymes.Citation2–Citation4 CaMKII isoform expression in the brain is developmentally and spatially regulated in vivo and in vitro. In the cerebral cortex CaMKIIβ expression is detected embryonically, while CaMKIIα expression is postnatal.Citation5–Citation7 CaMKII isoforms share 70∼90% sequence homology and consist of a catalytic, regulatory, variable and oligomerization domains.Citation8 The variable domain of CaMKII is alternatively spliced which alters subcellular localization and enzymatic attributes of the enzyme.Citation9,Citation10 In the variable domain of CaMKIIβ lies a F-actin binding domain that targets expressed CaMKIIβ to actin filaments in stress fibers and dendrite spines.Citation11–Citation13 We recently found that endogenous CaMKIIβ was highly enriched in a unique F-actin rich structure in developing cortical neurons.Citation14 This subcellular localization was pronounced and specific to CaMKIIβ ().
In addition to variable regions among the CaMKII isoforms, oligomerization is important for proper CaMKII localization. For example, monomeric CaMKIIβ, which lacks an oligomerization domain, does not colocalize with F-actin even though the F-actin binding domain is intact.Citation14 Although the F-actin binding domain in CaMKIIβ may be unique and required for CaMKIIβ targeting to F-actin, the domain itself is not sufficient to bind F-actin directly.Citation13,Citation14 CaMKIIδ binds to F-actin likely via a non-variable domain of the δ isoform that also requires oligomerization.Citation15,Citation16 This further emphasizes that proper assembly of the oligomer may be crucial for formation of a secondary structure which allows CaMKIIβ to anchor to actin filaments. In addition, all four isoforms of CaMKII can freely hetero-oligomerize via their oligomerization domains and the isoform composition of CaMKII influences enzyme localization.Citation9,Citation13,Citation17 For example two CaMKIIβ subunits are sufficient for targeting the holoenzyme to cortical F-actin.Citation13
CaMKII is readily activated in response to fluctuations of cellular calcium concentrations. Calcium induces calcium/calmodulin binding followed by two phosphorylation events.Citation18 The first of these is phosphorylation of T286 (in α) or T287 (in β, γ and δ) by a neighboring calcium/calmodulin-bound subunit. T286/7 autophosphorylated CaMKII is enzymatically active, but a subsequent calcium/calmodulin-independent phosphorylation at T305/306 suppresses the total enzymatic activity. The calcium/calmodulin-binding site of CaMKIIβ is adjacent to the F-actin binding domain. The activation of CaMKIIβ via calcium/calmodulin binding likely masks the F-actin binding site and therefore, competes with F-actin binding for CaMKIIβ. We used KN93, which binds to the calcium/calmodulin binding site of CaMKII, and demonstrated that calcium/calmodulin binding was required to dissociate CaMKIIβ from F-actin.Citation14 We further confirmed this by examining a mutant that cannot bind calcium/calmodulin, CaMKIIβ-A303R, and found that it bound F-actin similarly to wild-type CaMKIIβ. Likewise kinase and phosphorylation deficient mutants associated with F-actin to the same degree as wild-type CaMKIIβ whereas activated CaMKIIβ dissociated from F-actin.Citation14 This data indicates that even though a kinase and capable of phosphorylating actinCitation19 the kinase activity of CaMKIIβ was not required for F-actin binding. Our findings are consistent with biochemical studies indicating that dissociation of CaMKIIβ from F-actin requires calcium/calmodulin binding, but not kinase activity.Citation11,Citation12,Citation14
We found that wild-type, calcium/calmodulin binding deficient, and kinase deficient CaMKIIβ increased the prominence of F-actin rich structures whereas active CaMKIIβ did not. This ability of CaMKIIβ to promote the F-actin rich structures was directly tied to CaMKIIβ binding to F-actin. When CaMKIIβ—F-actin binding was tight, F-actin rich structures were more prominent and when binding was weak, prominence decreased.Citation14 This strong binding of CaMKIIβ to actin filaments and the loss of the F-actin rich structures after disruption of CaMKIIβ—F-actin binding are consistent with a role for CaMKIIβ in actin stabilization and bundling in vivo and in vitro.Citation19,Citation20
In summary, our work suggests that it is CaMKIIβ binding to F-actin that is the key to CaMKIIβ's ability to regulate actin filament stability. Although several kinases have been shown to bind F-actin and regulate actin dynamics, CaMKIIβ is unique in that it regulates the cytoskeleton independent of its kinase activity. CaMKIIβ's dual function, structural and enzymatic, expands it's role in cellular signaling, and suggests CaMKIIβ, and by inference other enzymes, lacks a purely inactive or nonfunctional state.
Figures and Tables
Figure 1 CaMKIIβ(red) highly colocalizes with F-actin (green) in discrete F-actin rich structures in an embryonic cortical neuron. These F-actin rich protrusions are enriched around or underneath the cell body. CaMKIIβ binding to F-actin is regulated by calcium signals and is important for the F-actin filament stability. Image courtesy of Yu-Chih Lin and Lori Redmond.
Addendum to:
References
- Tombes RM, Faison MO, Turbeville JM. Organization and evolution of multifunctional Ca2+/CaM-dependent protein kinase genes. Gene 2003; 322:17 - 31
- Bennett MK, Erondu NE, Kennedy MB. Purification and characterization of a calmodulin-dependent protein kinase that is highly concentrated in brain. J Biol Chem 1983; 258:12735 - 12744
- Erondu NE, Kennedy MB. Regional distribution of type II Ca2+/calmodulin-dependent protein kinase in rat brain. J Neurosci 1985; 5:3270 - 3277
- Brocke L, Chiang LW, Wagner PD, Schulman H. Functional implications of the subunit composition of neuronal CaM kinase II. J Biol Chem 1999; 274:22713 - 22722
- Bayer KU, Lohler J, Schulman H, Harbers K. Developmental expression of the CaM kinase II isoforms: ubiquitous gamma- and delta-CaM kinase II are the early isoforms and most abundant in the developing nervous system. Brain Res Mol Brain Res 1999; 70:147 - 154
- Karls U, Muller U, Gilbert DJ, Copeland NG, Jenkins NA, Harbers K. Structure, expression and chromosome location of the gene for the beta subunit of brain-specific Ca2+/calmodulin-dependent protein kinase II identified by transgene integration in an embryonic lethal mouse mutant. Mol Cell Biol 1992; 12:3644 - 3652
- Sahyoun N, LeVine H 3rd, Burgess SK, Blanchard S, Chang KJ, Cuatrecasas P. Early postnatal development of calmodulin-dependent protein kinase II in rat brain. Biochem Biophys Res Commun 1985; 132:878 - 884
- Lin CR, Kapiloff MS, Durgerian S, Tatemoto K, Russo AF, Hanson P, et al. Molecular cloning of a brain-specific calcium/calmodulin-dependent protein kinase. Proc Natl Acad Sci USA 1987; 84:5962 - 5966
- Griffith LC, Lu CS, Sun XX. CaMKII, an enzyme on the move: regulation of temporospatial localization. Mol Interv 2003; 3:386 - 403
- Hudmon A, Schulman H. Neuronal CA2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annu Rev Biochem 2002; 71:73 - 510
- Fink CC, Bayer KU, Myers JW, Ferrell JE Jr, Schulman H, Meyer T. Selective regulation of neurite extension and synapse formation by the beta but not the alpha isoform of CaMKII. Neuron 2003; 39:283 - 297
- Shen K, Meyer T. Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science 1999; 284:162 - 166
- Shen K, Teruel MN, Subramanian K, Meyer T. CaMKIIbeta functions as an F-actin targeting module that localizes CaMKIIalpha/beta heterooligomers to dendritic spines. Neuron 1998; 21:593 - 606
- Lin YC, Redmond L. CaMKII{beta} binding to stable F-actin in vivo regulates F-actin filament stability. Proc Natl Acad Sci USA 2008;
- Easley CA, Faison MO, Kirsch TL, Lee JA, Seward ME, Tombes RM. Laminin activates CaMK-II to stabilize nascent embryonic axons. Brain Res 2006; 1092:59 - 68
- Caran N, Johnson LD, Jenkins KJ, Tombes RM. Cytosolic targeting domains of gamma and delta calmodulin-dependent protein kinase II. J Biol Chem 2001; 276:42514 - 42519
- Lantsman K, Tombes RM. CaMK-II oligomerization potential determined using CFP/YFP FRET. Biochim Biophys Acta 2005; 1746:45 - 54
- Colbran RJ, Brown AM. Calcium/calmodulin-dependent protein kinase II and synaptic plasticity. Curr Opin Neurobiol 2004; 14:318 - 327
- O'Leary H, Lasda E, Bayer KU. CaMKIIbeta association with the actin cytoskeleton is regulated by alternative splicing. Mol Biol Cell 2006; 17:4656 - 4665
- Okamoto K, Narayanan R, Lee SH, Murata K, Hayashi Y. The role of CaMKII as an F-actin-bundling protein crucial for maintenance of dendritic spine structure. Proc Natl Acad Sci USA 2007; 104:6418 - 6423