Abstract
Agrobacterium genetically transforms its hosts by transferring a segment of DNA (T-DNA) into the host cell and integrating it into the host genome. Integration requires a close interaction between T-DNA, which is packaged into a nucleoprotein complex (T-complex) by bacterial virulence (Vir) proteins, and the host chromatin. This interaction is facilitated by the host protein VIP 1, which binds both to the major protein component of the T-complex, VirE2, and to the core histones. Recently, VIP1 has been demonstrated to mediate the interaction between plant nucleosomes and VirE2-DNA complexes (i.e., synthetic T-complex-like structures) in vitro. Here, we discuss major implications of these observations—such as the possible role of core histone modifications, proteasomal uncoating of the T-complex mediated by the bacterial F-box protein VirF, and the need for changes in chromatin structure to render it accessible to the T-DNA integration—for the process of chromatin targeting of foreign DNA and its integration into the eukaryotic genome.
Introduction
Agrobacterium tumefaciens is a soil phytopathogen with a unique ability to transfer a segment of its DNA (T-DNA) into the genome of eukaryotic cells. Although plants are the natural hosts for Agrobacterium, this microorganism can also genetically transform a wide range of other eukaryotic species, from fungiCitation1,Citation2 to human cells.Citation3 Expression of the T-DNA genes in host plant cells results in uncontrolled cell proliferation due to modification of growth regulator balance and synthesis of opines, molecules that can be used by the bacteria as a source of carbon and nitrogen (reviewed in ref. Citation4). T-DNA is transported from the bacterium to the host cell as a single-stranded DNA (ssDNA) molecule (T-strand).Citation5
During its travel to the host genome, the T-strand is first transported into the host cell cytoplasm via a type IV secretion system,Citation6 imported in the host cell nucleus via an importin α-dependent pathway,Citation7–Citation10 and finally targeted to host chromatin before integration.Citation6,Citation7,Citation11,Citation12 It is thought that the T-strand travels within the host cell as a nucleoprotein complex (T-complex) with bacterial virulence (Vir) proteins as well as host proteins. Briefly, one molecule of VirD2 is covalently attached at the 5′ end of the T-strand, and numerous VirE2 molecules coat the T-strand over its entire length.Citation12 Nuclear import of the T-complex is mediated by VirD2, which binds directly to the host importin α,Citation13 and by VirE2, which does not bind importin α efficiently,Citation14 but interacts with VIP1 (VirE2 interacting protein 1)Citation15 which then binds importin α, acting as a molecular adaptor between VirE2 and the importin α-dependent nuclear import machinery.Citation14,Citation16
While the processes of the T-strand formation, its export to the host cell, and nuclear import of the T-complex are relatively well studied, the mechanism by which the T-complex is targeted to and associates with the host cell chromatin remains enigmatic. The cell nucleus is a complex organelle within which macromolecular traffic is tightly regulated.Citation17,Citation18 It is thus likely that a specific mechanism exists to target a large structure, such as the T-complex, to the chromatin. To gain an insight into this mechanism, we explored in vitro interactions between plant nucleosomes, VIP1 and other components of the T-complex.Citation19
Requirement for a Link Between the Host Chromatin and the Invading T-Complex
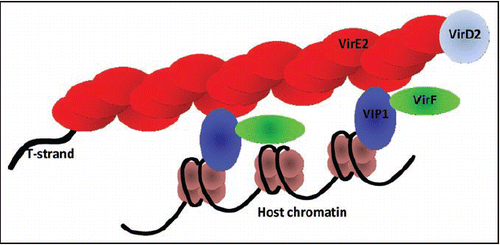
Integration of the T-DNA into host genome obviously requires a close interaction with the chromatin. The ability of VIP1 to bind core histones,Citation20,Citation21 and interact with VirE2 and, therefore, the T-complex,Citation15 makes it an attractive candidate for this role of a molecular link between the T-complex and the host chromatin. Our resultsCitation19 indeed demonstrate that VIP1, besides binding strongly and specifically to nucleosomes, is able to mediate in vitro interaction between nucleosomes and VirE2 as well as between nucleosomes and VirE2-ssDNA complexes, i.e., synthetic T-complex-like structures (). These data are consistent with the demonstrated role of VIP1 in planta as a specific enhancer of the Agrobacterium-mediated gene transfer.Citation16 In respect to its roles in Agrobacterium infection, VIP1 contains two functional domains: an N-terminal domain that interacts with VirE2 and a C-terminal domain that binds core histones in vivo.Citation20 Similarly, in vitro, the N-terminal domain of VIP1, while still able to bind VirE2, does not mediate the nucleosome-VirE2 interaction.Citation19 Collectively, these observations establish the role of VIP1 as a molecular bridge between nucleosomes and VirE2. However, the role in chromatin targeting of additional plant factors interacting with the T-complex—e.g., VirD2-binding proteinsCitation22,Citation23 or another VirE2-binding protein, VIP2,24—cannot be ruled out. VIP1, therefore, may be only one of a series of factors that can mediate the T-complex interaction with the host chromatin prior to and during the T-DNA integration.
Is There a Role for Histone Modifications During the T-Complex Chromatin Targeting?
We did not detect preferential binding to VIP1 to modified histones (acetylated histone H3 and lysine 4-methylated histone H3) which represents markers of transcriptionally active chromatin regions.Citation19 This is consistent with recent observations that T-DNA integrates randomly, irrespective of the transcriptional state of the chromatin.Citation25,Citation26 However, other types of histone covalent modifications might be involved in targeting the T-complex to the integration sites. For example, the T-DNA integration is thought to occur preferentially at double-stranded DNA breaks (DSBs),Citation27–Citation29 and non-homologous end joining (NHEJ) machinery is involved in the integration process.Citation30
A site-specific phosphorylation at serine-139 of histone H2A, which specifically occurs in the DSB regions, is necessary for recruitment and binding of the NHEJ proteins.Citation31 In animals and yeast, this phosphorylation involves a specific variant of H2A, H2AX, whereas, in plants, the corresponding H2A variants, termed HTA3 and HTA5, were also shown to undergo the serine-139 phosphorylation.Citation32 It is tempting to speculate that phosphorylated HTA3 and HTA5 may bind a component of the T-complex, such as VIP1, with a higher affinity than their non-phosphorylated forms, thereby “attracting” the T-complex to the DSB sites. Our initial experiments using HTA3 and its phosphorylation-mimicking mutant S139D, did not detect such preferential binding of VIP1 (Lacroix B and Citovsky V, unpublished). However, the negatively charged amino acid substitution may not have faithfully reproduced the full spectrum of the effects of HTA3 phosphorylation on chromatin at the DSB site, or other protein factors may be required for modulation of VIP1 binding.
When Does the T-Complex Uncoating Occur?
Before integration can take place, the proteins coating the T-strand must be removed. Our earlier dataCitation33 indicate that this is achieved by proteasomal degradation of VirE2 and VIP1 and this degradation is mediated by a bacterial F-box protein, VirF, which is translocated into host cellCitation34 and recognizes VIP1 as a substrate for the SCFVirF pathway.Citation33 Yet, it remained unknown whether VirF can recognize the T-complex while it is already associated with the host chromatin. Our new dataCitation19 indicate that VirF can bind VIP1 when the latter is still attached both to nucleosomes and VirE2-ssDNA complexes. Thus, VirF most likely functions at the stage when the T-complex is bound to the chromatin via VIP1.
But is there a mechanism to prevent “premature” uncoating that could occur before chromatin targeting? Potentially, additional host proteins or bacterial virulence proteins exported to the host cell may prevent VirF binding to VIP1 and/or to the ASK1 component of the SCFVirF complex,Citation33,Citation35 until the T-complex is in contact with chromatin. Moreover, because T-DNA can be integrated as double-stranded DNA, removal of the coating proteins may be necessary before the second-strand synthesis can occur. It is still unknown whether the second-strand synthesis occurs before or after the T-complex associates with the chromatin. Our resultsCitation19 suggest that the T-complex first interacts with the chromatin via VIP1, then the T-complex is uncoated via the SCFVirF pathway, exposing the T-strand for the second-strand synthesis and integration, which may represent coupled events.
Chromatin Form “Susceptible” for the T-DNA Integration
Another interesting aspect of the T-DNA integration process is that not only the T-strand must be uncoated of its associated proteins, but also the target host DNA must be made accessible to the integration machinery, potentially by decondensing, or even partially unpacking the chromatin. Although earlier data suggested that T-DNA integrates mainly into active (decondensed) regions of the chromatin,Citation36,Citation37 more recent evidence indicates that the integration is truly random and independent of active or inactive state of the target chromatin.Citation25 Yet, even if the T-DNA can integrate equally well into both euchromatin and heterochromatin, at least a local and transient decondensation of the chromatin is most likely required for efficient integration. While little is known about how the host chromatin is perturbed during the T-DNA integration, several potential scenarios can be envisioned.
The T-complex can be targeted preferentially to the chromatin regions, which are already present in a form “susceptible” to integration. For example, DSBs, into which the T-DNA preferentially integratesCitation27–Citation29 and in which the DNA may be already exposed to the repair machinery, may represent such sites. Alternatively, a change of the chromatin structure may be transiently induced by one of the protein components of the T-complex itself during its interaction with chromatin. For example, VirF can bind VIP1 while VIP1 is attached both to nucleosomes and to the VirE2-ssDNA complexCitation19 (see ). If, under these conditions, VirF promotes degradation of VirE2 attached to VIP1,Citation33 it may, in the same manner, induce degradation of the core histones which are also bound to VIP1.Citation19–Citation21 Such local destabilization of the core histones would create a chromatin region favorable to the T-DNA integration.
Conclusions
Increasing evidence indicates that Agrobacterium has evolved to subvert many diverse cellular systems for its own needs.Citation7,Citation38 One of the best examples of this strategy is the reliance of Agrobacterium on the host VIP1 protein. While the endogenous function of VIP1 is still unclear, this basic domain-leucine zipper (bZIP) protein may act as a transcription factorCitation15 involved in plant defense.Citation38 As a transcription factor, VIP1 is able to enter the cell nucleus and reach the chromatin. As a defense-related protein, the activity of VIP1 is enhanced following Agrobacterium infection.Citation39 Thus, it makes biological sense that Agrobacterium takes advantage of VIP1, diverting it from its native function to acting as a link between the T-complex and cellular pathways (e.g., nuclear import, chromatin targeting, and proteasomal degradation) important for the infection.
It is important to note, however, that Agrobacterium does not rely on VIP1 exclusively. For example, VIP1 is a plant-specific protein, not found in non-plant species.Citation15,Citation40,Citation41 Yet, Agrobacterium can infect non-plant eukaryotic cells,Citation42 at least under laboratory conditions. Thus, different Agrobacterium species most likely have evolved alternative pathways for infection of diverse eukaryotic hosts. For example, Agrobacterium tumefaciens encodes a VirE3 protein that is exported into the host cellsCitation43 and, at least partially, mimics the VIP1 function.Citation44 In addition, some strains of Agrobacterium rhizogenes do not encode VirE2, but instead produce the GALLS protein, which fulfills the VirE2 functions and likely uses a VIP1-independent pathways for nuclear import, interaction with chromatin, and integration.Citation45 Overall, however, while Agrobacterium utilizes largely its own molecular systems for introduction of DNA and proteins into the host cells, it increasingly exploits the host factors for subsequent stages of genetic transformation, especially those, such as chromatin targeting and uncoating of the T-complex and subsequent T-DNA integration, that take place in the host cell nucleus.
Abbreviations
| T-DNA | = | transferred DNA |
| VIP1 | = | VirE2-interacting protein 1 |
| ssDNA | = | single strand DNA |
| NHEJ | = | non homologous end joining |
References
- Piers KL, Heath JD, Liang X, Stephens KM, Nester EW. Agrobacterium tumefaciens-mediated transformation of yeast. Proc Natl Acad Sci USA 1996; 93:1613 - 1618
- de Groot MJ, Bundock P, Hooykaas PJJ, Beijersbergen AG. Agrobacterium tumefaciens-mediated transformation of filamentous fungi [published erratum appears in Nat Biotechnol 16, 1074 (1998)]. Nat Biotechnol 1998; 16:839 - 842
- Kunik T, Tzfira T, Kapulnik Y, Gafni Y, Dingwall C, Citovsky V. Genetic transformation of HeLa cells by Agrobacterium. Proc Natl Acad Sci USA 2001; 98:1871 - 1876
- Gelvin SB. Agrobacterium-mediated plant transformation: the biology behind the “gene-jockeying” tool. Microbiol Mol Biol Rev 2003; 67:16 - 37
- Stachel SE, Timmerman B, Zambryski PC. Generation of single-stranded T-DNA molecules during the initial stages of T-DNA transfer for Agrobacterium tumefaciens to plant cells. Nature 1986; 322:706 - 712
- Christie PJ. Type IV secretion: the Agrobacterium VirB/D4 and related conjugation systems. Biochim Biophys Acta 2004; 1694:219 - 234
- Citovsky V, Kozlovsky SV, Lacroix B, Zaltsman A, Dafni M, Vyas S, Tovkach A, Tzfira T. Biological systems of the host cell involved in Agrobacterium infection. Cell Microbiol 2007; 9:9 - 20
- Lacroix B, Li J, Tzfira T, Citovsky V. Will you let me use your nucleus? How Agrobacterium gets its T-DNA expressed in the host plant cell. Can J Physiol Pharmacol 2006; 84:333 - 345
- Ziemienowicz A, Merkle T, Schoumacher F, Hohn B, Rossi L. Import of Agrobacterium T-DNA into plant nuclei: two distinct functions of VirD2 and VirE2 proteins. Plant Cell 2001; 13:369 - 384
- Bhattacharjee S, Lee LY, Oltmanns H, Cao H, Veena Cuperus J, Gelvin SB. IMPa-4, an Arabidopsis importin alpha isoform, is preferentially involved in Agrobacterium-mediated plant transformation. Plant Cell 2008; 20:2661 - 2680
- Gelvin SB. Agrobacterium and plant genes involved in T-DNA transfer and integration. Annu Rev Plant Physiol Plant Mol Biol 2000; 51:223 - 256
- Tzfira T, Citovsky V. Partners-in-infection: host proteins involved in the transformation of plant cells by Agrobacterium. Trends Cell Biol 2002; 12:121 - 129
- Ballas N, Citovsky V. Nuclear localization signal binding protein from Arabidopsis mediates nuclear import of Agrobacterium VirD2 protein. Proc Natl Acad Sci USA 1997; 94:10723 - 10728
- Citovsky V, Kapelnikov A, Oliel S, Zakai N, Rojas MR, Gilbertson RL, Tzfira T, Loyter A. Protein interactions involved in nuclear import of the Agrobacterium VirE2 protein in vivo and in vitro. J Biol Chem 2004; 279:29528 - 29533
- Tzfira T, Vaidya M, Citovsky V. VIP1, an Arabidopsis protein that interacts with Agrobacterium VirE2, is involved in VirE2 nuclear import and Agrobacterium infectivity. EMBO J 2001; 20:3596 - 3607
- Tzfira T, Vaidya M, Citovsky V. Increasing plant susceptibility to Agrobacterium infection by overexpression of the Arabidopsis VIP1 gene. Proc Natl Acad Sci USA 2002; 99:10435 - 10440
- Phair RD, Misteli T. High mobility of proteins in the mammalian cell nucleus. Nature 2000; 404:604 - 609
- Miyoshi D, Sugimoto N. Molecular crowding effects on structure and stability of DNA. Biochimie 2008; 90:1040 - 1051
- Lacroix B, Loyter A, Citovsky V. Association of the Agrobacterium T-DNA-protein complex with plant nucleosomes. Proc Nat Acad Sci USA 2008; 105:15429 - 15434
- Li J, Krichevsky A, Vaidya M, Tzfira T, Citovsky V. Uncoupling of the functions of the Arabidopsis VIP1 protein in transient and stable plant genetic transformation by Agrobacterium. Proc Natl Acad Sci USA 2005; 102:5733 - 5738
- Loyter A, Rosenbluh J, Zakai N, Li J, Kozlovsky SV, Tzfira T, Citovsky V. The plant VirE2 interacting protein 1. A molecular link between the Agrobacterium T-complex and the host cell chromatin?. Plant Physiol 2005; 138:1318 - 1321
- Deng W, Chen L, Wood DW, Metcalfe T, Liang X, Gordon MP, Comai L, Nester EW. Agrobacterium VirD2 protein interacts with plant host cyclophilins. Proc Natl Acad Sci USA 1998; 95:7040 - 7045
- Bakó L, Umeda M, Tiburcio AF, Schell J, Koncz C. The VirD2 pilot protein of Agrobacterium-transferred DNA interacts with the TATA box-binding protein and a nuclear protein kinase in plants. Proc Natl Acad Sci USA 2003; 100:10108 - 10113
- Anand A, Krichevsky A, Schornack S, Lahaye T, Tzfira T, Tang Y, Citovsky V, Mysore KS. Arabidopsis VIRE2 INTERACTING PROTEIN2 Is required for Agrobacterium T-DNA integration in plants. Plant Cell 2007; 19:1695 - 1708
- Kim SI, Veena Gelvin SB. Genome-wide analysis of Agrobacterium T-DNA integration sites in the Arabidopsis genome generated under non-selective conditions. Plant J 2007; 51:779 - 791
- Gelvin SB, Kim SI. Effect of chromatin upon Agrobacterium T-DNA integration and transgene expression. Biochim Biophys Acta 2007; 1769:409 - 420
- Salomon S, Puchta H. Capture of genomic and T-DNA sequences during double-strand break repair in somatic plant cells. EMBO J 1998; 17:6086 - 6095
- Tzfira T, Frankmen L, Vaidya M, Citovsky V. Site-specific integration of Agrobacterium T-DNA via double-stranded intermediates. Plant Physiol 2003; 133:1011 - 1023
- Chilton MD, Que Q. Targeted integration of T-DNA into the tobacco genome at double-strand breaks: new insights on the mechanism of T-DNA integration. Plant Physiol 2003; 133:956 - 965
- Tzfira T, Li J, Lacroix B, Citovsky V. Agrobacterium T-DNA integration: molecules and models. Trends Genet 2004; 20:375 - 383
- Foster ER, Downs JA. Histone H2A phosphorylation in DNA double-strand break repair. FEBS J 2005; 272:3231 - 3240
- Rybaczek D, Maszewski J. Phosphorylation of H2AX histones in response to double-strand breaks and induction of premature chromatin condensation in hydroxyurea-treated root meristem cells of Raphanus sativus, Vicia faba and Allium porrum. Protoplasma 2007; 230:31 - 39
- Tzfira T, Vaidya M, Citovsky V. Involvement of targeted proteolysis in plant genetic transformation by Agrobacterium. Nature 2004; 431:87 - 92
- Vergunst AC, Schrammeijer B, den Dulk-Ras A, de Vlaam CMT, Regensburg-Tuink TJ, Hooykaas PJJ. VirB/D4-dependent protein translocation from Agrobacterium into plant cells. Science 2000; 290:979 - 982
- Schrammeijer B, Risseeuw E, Pansegrau W, Regensburg-Tuïnk TJG, Crosby WL, Hooykaas PJJ. Interaction of the virulence protein VirF of Agrobacterium tumefaciens with plant homologs of the yeast Skp1 protein. Curr Biol 2001; 11:258 - 262
- Szabados L, Kovács I, Oberschall A, Ábrahám E, Kerekes I, Zsigmond L, Nagy R, Alvarado M, Krasovskaja I, Gál M, Berente A, Rédei GP, Haim AB, Koncz C. Distribution of 1000 sequenced T-DNA tags in the Arabidopsis genome. Plant J 2002; 32:233 - 242
- Chen S, Jin W, Wang M, Zhang F, Zhou J, Jia Q, Wu Y, Liu F, Wu P. Distribution and characterization of over 1000 T-DNA tags in rice genome. Plant J 2003; 36:105 - 113
- Djamei A, Pitzschke A, Nakagami H, Rajh I, Hirt H. Trojan horse strategy in Agrobacterium transformation: abusing MAPK defense signaling. Science 2007; 318:453 - 456
- Dietz GP, Bahr M. Delivery of bioactive molecules into the cell: the Trojan horse approach. Mol Cell Neurosci 2004; 27:85 - 131
- Rhee Y, Gurel F, Gafni Y, Dingwall C, Citovsky V. A genetic system for detection of protein nuclear import and export. Nat Biotechnol 2000; 18:433 - 437
- Tzfira T, Citovsky V. Comparison between nuclear import of nopaline- and octopine-specific VirE2 protein of Agrobacterium in plant and animal cells. Mol Plant Pathol 2001; 2:171 - 176
- Lacroix B, Tzfira T, Vainstein A, Citovsky V. A case of promiscuity: Agrobacterium's endless hunt for new partners. Trends Genet 2006; 22:29 - 37
- Schrammeijer B, den Dulk-Ras A, Vergunst AC, Jurado Jácome E, Hooykaas PJJ. Analysis of Vir protein translocation from Agrobacterium tumefaciens using Saccharomyces cerevisiae as a model: evidence for transport of a novel effector protein VirE3. Nucleic Acids Res 2003; 31:860 - 868
- Lacroix B, Vaidya M, Tzfira T, Citovsky V. The VirE3 protein of Agrobacterium mimics a host cell function required for plant genetic transformation. EMBO J 2005; 24:428 - 437
- Hodges LD, Cuperus J, Ream W. Agrobacterium rhizogenes GALLS protein substitutes for Agrobacterium tumefaciens single-stranded DNA-binding protein VirE2. J Bacteriol 2004; 186:3065 - 3077