Abstract
Neuregulin-1 (NRG-1) and its receptor ErbB4 are genetically linked with schizophrenia, a complex developmental disorder of high heritability but unknown etiology that has been proposed to result from deficits in functional connectivity and synaptic plasticity. Based on pharmacological evidence, disbalances in dopaminergic and glutamatergic transmission systems are believed to contribute to its pathophysiology, but genetic data supporting a causative role for either are sparse. Stimulation of NRG-1/ErbB4 signaling inhibits or reverts hippocampal long-term potentiation at glutamatergic synapses between Schaeffer collateral afferents and CA1 pyramidal neurons (SC->CA1). We have recently demonstrated that NRG-1 regulates glutamatergic plasticity by rapidly increasing extracellular hippocampal dopamine levels and activation of D4 dopamine receptors (D4R). These new findings position NRG-1/ErbB4 signaling pathway at the crossroads between dopaminergic and glutamatergic neurotransmission and offer novel ways to consolidate genetic, functional and pharmacological data toward a better understanding of the etiological processes underlying schizophrenia, and the role of NRG-1 for normal synaptic function and plasticity. The currently available data suggest that hippocampal interneurons might play a crucial role in mediating NRG-1 induced depotentiation. This interpretation is in line with other evidence pointing towards an involvement of GABAergic cells in the etiology of schizophrenia.
Activation of NRG-1/ErbB4 Signaling Recruits a Dopaminergic Pathway to Regulate Early LTP at Glutamatergic CA1 Synapses
At SC→CA1 synapses, LTP is rapidly depotentiated by acute administration of NRG-1 during a labile period of ∼30 min immediately following its induction.Citation1 In addition, NRG-1 can prevent the manifestation of LTP if applied prior to induction.Citation2,Citation3 LTP can also be reversed by a brief train of electrical pulses at theta frequency (theta-pulse stimuli, TPS), and this TPS-mediated depotentiation is blocked by inhibitors of ErbB signaling, suggesting an involvement of the NRG/ErbB signaling pathway.Citation1
Mechanistically, LTP depotentiation by NRG-1 represents the reversal of synaptic strength back to pre-LTP levels, and is mediated via the internalization of synaptic AMPA receptors containing the GluR1 subunit. Although previous work firmly established the inhibitory effects of NRG-1/ErbB signaling on LTP, the pathway leading from ErbB receptor activation to the removal of AMPA receptors selectively from potentiated synapses was unclear. On the other hand, mice heterozygous for NRG-1 display abnormal pre-pulse inhibition of the startle response, a behavioral abnormality that is also found in schizophrenic patients and that can be alleviated by treatment with the antipsychotic clozapine,Citation4 that primarily targets D2-type dopamine receptors.Citation5,Citation6 This prompted us to investigate a possible involvement of the dopamine system in mediating NRG-1-induced depotentiation.
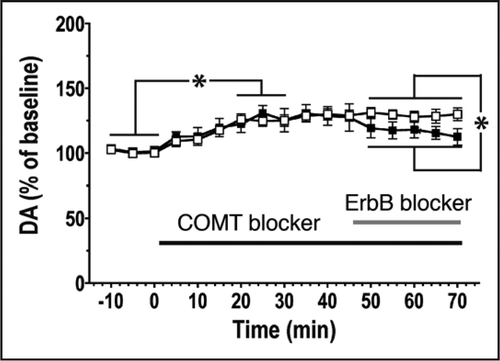
We found that perfusion of the dorsal hippocampus of behaving animals with NRG-1 causes a rapid (<2 min) and dramatic (∼3-fold) increase in extracellular dopamine levels that lasted for about 15 minutes.Citation7 Conversely, pharmacological blockade of ErbB receptors produces a small but significant decrease of dopamine levels, consistent with a role for endogenous NRG-1 signaling in the regulation of dopamine (). We then investigated the involvement of dopamine receptors in LTP reversal and identified through the use of selective agonists and antagonists, as well as KO mice, the D4 dopamine receptor (D4R) as necessary and sufficient to trigger LTP depotentiation in response to NRG-1.Citation7 Moreover, TPS are not effective in attenuating LTP in D4R-knockout mice, further corroborating the notion that TPS and NRG-1 share a common pathway. Consistent with the idea that D4R could be an intermediate downstream target of NRG-1/ErbB4 signaling, we also showed that in cultured hippocampal neurons expressing D4R and treated to undergo a chemical form of LTP, D4R activation causes the internalization of surface GluR1-containing AMPA receptors, thus recapitulating the response in acute hippocampal slices.Citation7 Our slice recordings indicate that the depotentiating effects of NRG-1 and D4R agonists are local to CA1, since results were not different when CA3 was separated from CA1. This result is consistent with the distribution of dopamine fibers in hippocampus that innervate the subiculum and adjacent CA1 but not CA3 (reviewed in ref. Citation8), and with the presence of D4R mRNA in CA1/CA2.Citation7
Complementary Roles of D1-Type and D4 Dopamine Receptors during LTP
Numerous studies have shown that D1/D5 receptors facilitate early-phase LTPCitation9 and have an important role in the consolidation of late-phase LTP in vitroCitation10,Citation11 and in vivo.Citation12,Citation13 In contrast, the role of D2-type receptors in LTP has been much less well defined. All dopamine receptors are G-protein coupled and modulate the production of cyclic AMP. Since D1-type receptors and D2-type receptors stimulate or inhibit adenylate cyclase activity, respectively, they may be expected to exert opposing effects on LTP. Interestingly, the D2/D3R antagonist domperidone has been shown to prevent late-phase LTP,Citation14,Citation15 however, this effect required several hours to occur and thus likely reflects a different mechanism, since the specific activation of D4R in our study resulted in almost immediate depotentiation.Citation7 Our results show that local application of NRG-1 increases dopamine release in the hippocampus in vivo. Frey et al. showed that LTP-inducing 100 Hz tetanization of the SC→CA1 pathway temporarily increases the release of [14C] from sections that were preloaded with [14C]-dopamine.Citation14 However, while tetanus-elicited dopamine release could explain the stabilizing effects of dopamine signaling on late-phase LTP, it is unlikely to account for the rapid depotentiating effects of NRG-1 based on differences in timing and sign of action (pro-LTP vs. anti-LTP).
Location, Location, Location
Although conceptually the recruitment of the dopamine system by NRG-1 elegantly links ErbB4 signaling and glutamatergic plasticity, it is currently not clear where within the circuitry of the hippocampus the critical ‘players’ are located. It appears reasonable to assume that the D4R is expressed on CA1 principal neurons at or near SC→CA1 synapses in the stratum radiatum to locally trigger the reversal of LTP at potentiated sites (depotentiation by NRG-1 is homosynaptic; see ref. Citation1), based on our in vitro data showing that direct activation of D4R can trigger AMPA receptor internalization in transfected hippocampal neurons. On the other hand, D4R immunoreactivity in the primate hippocampus was shown to be high in some GABAergic interneurons,Citation16 suggesting that D4R activation could somehow reverse LTP by modulating GABAergic function. It is important to note, however, that D4R mRNA and protein levels are exceedingly low in the hippocampus, and its expression in pyramidal cells and/or interneurons is far from established.Citation16–Citation18
The known spatial distribution of some crucial elements might be helpful, however, in assembling a network model: First, ErbB4 is expressed in numerous GABAergic interneurons across all layers (see below). Second, glutamatergic SC→CA1 synapses are located in the stratum radiatum. Third, the dopaminergic innervation of the hippocampus is low and spatially restricted to the subiculum and adjacent CA1, especially in the dorsal part where electrophysiological recordings of LTP are typically performed; mesohippocampal dopaminergic fibers in CA1 terminate in stratum oriens close to the alveus or in stratum lacunosum moleculare, essentially excluding strata pyramidale and radiatum.Citation8,Citation19 It is unclear if dopamine receptors located close to SC→CA1 synapses on pyramidal neurons could be directly activated by dopamine release from these terminals, even if considering the possibility of extrasynaptic volume transmission of dopamine as suggested in other brain areas such as the nucleus accumbens.Citation20 If dopamine receptors are present in the vicinity of dopaminergic fibers they could be located on distal portions of basal and apical pyramidal cell dendrites, on interneurons, or both. Finally, it is as of yet unclear whether hippocampal dopamine receptors are primarily postsynaptic or presynaptic, or whether cells co-express different types of dopamine receptors.
As for the location of ErbB4, direct receptor activation on dopamine terminals presents one conceivable way to promote release from afferents in the hippocampus. However, we did not find evidence for ErbB4 expression in dopamine neurons in the VTA or their afferent projections in the hippocampus. In contrast, the highest levels of ErbB4 mRNA and protein have consistently been observed in GABAergic interneurons, and these cells are good candidates to represent the proximate target for the effects of NRG-1 on glutamatergic plasticity in pyramidal cells. In agreement with this, NRG-1 has been shown to stimulate GABA release.Citation21
Interestingly, hypofunction of GABAergic interneurons, in particular of cells that express parvalbumin (PV), has been suggested to contribute to the etiology of schizophrenia.Citation22–Citation25 Basket and chandelier cells that provide powerful perisomatic inhibition control and time the population firing of principal glutamatergic neurons, thereby coordinating network activity. Acute activation of NRG-1/ErbB4 signaling strongly increases the power of kainate-induced gamma-oscillations in CA3 of the hippocampus, suggesting that it augments or synchronizes basket cell output.Citation26 However, it is not obvious how perisomatic inhibition of pyramidal cells could be linked to LTP reversal at their apical dendrites where SC→CA1 axons terminate. Of note, studies on the hippocampal distribution of ErbB4 unanimously show expression in numerous cells that are located in the apical strata radiatum and lacunosum moleculare, suggesting that ErbB4 expression in interneurons extends beyond perisomatic targeting cells (refs. Citation27 and Citation28; Neddens J and Buonanno A, unpublished). To understand if and how NRG-1/ErbB4 signaling regulates dopamine release via modulation of GABAergic function, it will be important to investigate in detail the extent to which ErbB4 is expressed in other interneuron subtypes that regulate and coordinate the flow of glutamatergic information through the hippocampal subfields, and to study mice with subtype-specific ablations of the receptor. Lastly, it will be imperative to identify the source of endogenous NRG that regulates plasticity at SC→CA1 synapses, and the conditions under which it triggers dopamine release (see below). Previous work suggests that NRG-1 can be released in an activity-dependent fashion, and that CA3 axons, perforant path fibers, and cholinergic afferents from the medial septum, are all potential sources.Citation29–Citation31
Implications for Schizophrenia
Glutamatergic, dopaminergic, GABAergic and cholinergic neurotransmitter systems have all been implicated in the etiology and pathophysiology of schizophrenia. Recently, a circuit-based framework of schizophrenia was proposed in which aberrant glutamatergic or cholinergic function reduces GABAergic control of principal neuron firing by parvalbumin-expressing basket and chandelier cells, thereby causing disinhibition of glutamatergic outflow from the hippocampus, increased neuronal activity in the VTA, and subsequent generation of a hyperdopaminergic state in the hippocampus.Citation32 Among the many genetic risk factors that have been implicated in this process, for the reasons mentioned above NRG-1 and ErbB4 seem uniquely positioned to link these neurotransmitter systems, in particular since ErbB4 is co-expressed in parvalbumin-positive interneurons.Citation25 However, whether NRG signaling causes dopamine release via a polysynaptic loop involving the VTA is presently unclear. While our dopamine measurements in live behaving animals are compatible with this idea, it should be noted that our electrophysiological experiments implicating D4R in NRG-mediated LTP reversal were performed in acute hippocampal slice preparations in which the VTA was removed,Citation7 thus favoring a local mechanism.
Historically, the NRG/ErbB signaling network is best known for its important role in the development and maturation of the peripheral and central nervous system, including the generation, migration and survival of inhibitory interneurons,Citation33–Citation35 synaptic maturation,Citation36,Citation37 and possibly the control of myelination of central axonsCitation38,Citation39 (but see ref. Citation40). In this way, NRG/ErbB signaling is involved in multiple neural processes potentially relevant to the etiology of schizophrenia, and alterations of which could conceivably increase the risk of developing the disorder later in adolescence.
It was recently shown that the schizophrenia-associated NRG-1 polymorphism SNP8NRG243177 correlates with impaired activation of the medial prefrontal and temporal cortex and affects IQ (refs. Citation41 and Citation42, but see ref. Citation43). However, it is still an open question whether hypo-or hyperactive NRG-1/ErbB4 signaling (or both) is associated with schizophrenia. Based on analyses of NRG-1 and ErbB4 mRNA levels in postmortem brains, increased expression of certain NRG-1 and ErbB4 isoforms was proposed in schizophrenic patients.Citation44,Citation45 Moreover, ErbB4 signaling after stimulation with exogenous NRG-1 peptide is enhanced in synaptic membrane preparations from postmortem brains of affected individuals.Citation46 Taken together, these findings appear to favor a model in which hyperfunction of NRG-1/ErbB4 signaling contributes to schizophrenia. On the other hand, behavioral abnormalities that are consistent with some of the positive symptoms associated with schizophrenia have been observed in mice heterozygous for NRG-1 or ErbB4, suggesting that reduced NRG-1/ErbB4 signaling could trigger schizophrenia-like behavior.Citation4 Therefore, while there is ample data to suggest a link between NRG-1/ErbB4 signaling and the manifestation of schizophrenia-associated behavior, it is evident that we are still a long way from understanding how, and on what time scale, alterations in NRG-1/ErbB4 signaling contribute to the impairment of cognitive processes observed in schizophrenia. In particular, it is unclear whether the changes in NRG-1/ErbB4 function and expression observed in patients and postmortem brain samples represent direct effects or compensatory responses to some other primary perturbation. For the same reasons, caution needs to be exercised in trying to equate findings derived from the analysis of genetic animal models with those obtained from acute manipulations of the neuregulin/ErbB signaling network. We are confident, however, that our recent findings will encourage new studies in humans and mouse models to further explore the causative role NRG plays in the regulation of dopaminergic and glutaminergic transmission in the normal brain and under pathological conditions. It should also be mentioned that several recent studies have pointed towards a functional connection between NRG and the nicotinic α7 acetylcholine receptor (α7R),Citation47–Citation49 the prospect of which is exciting since several lines of genetic and functional evidence point to an involvement of α7R with schizophrenia (reviewed in refs. Citation50 and Citation51).
Lastly, most studies have focused on the role of NRG-1 on synaptic function and plasticity. In this regard, it is important to emphasize, though, that the closely related NRG-2 is highly expressed in the adult brain and therefore conceivably serves as an important endogenous ligand for ErbB4, while NRG-1 expression is highest during neural development.Citation52 In contrast to NRG-1 mutant mice that die during early embryogenesis, NRG-2 deficient mice are viable and could therefore serve as a useful model to analyze the effects of impaired NRG/ErbB4 signaling on hippocampal function in the adult animal.Citation53
Figures and Tables
Figure 1 Blockade of endogenous NRG/ErbB signaling reduces dopamine release in the dorsal hippocampus of live behaving rats. Catechol-O-methyl transferase (COMT) activity was blocked with 100 nM Ro-41-0960 to prevent dopamine degradation and to analyze the effects of ErbB receptor inhibition (10 µM PD158780) on dopamine release. Dopamine levels rise steadily over 25 min following the onset of Ro-41-0960 infusion, consistent with the predominant role of enzymatic degradation in the clearance of extracellular dopamine in the hippocampus.Citation7 However, after additional infusion of the ErbB receptor inhibitor (solid squares), dopamine levels decrease significantly compared to controls that did not receive the blocker (open squares). Therefore, ErbB receptor activation is involved in the regulation of endogenous dopamine release. *p < 0.05 (2-way ANOVA). Data represent the mean ± SEM (n = 5 for both groups).
Acknowledgements
The authors would like to acknowledge the Eunice Kennedy Shriver National Institute of Child Health and Human Development for their financial support.
References
- Kwon OB, Longart M, Vullhorst D, Hoffman DA, Buonanno A. Neuregulin-1 reverses long-term potentiation at CA1 hippocampal synapses. J Neurosci 2005; 25:9378 - 9383
- Huang YZ, Won S, Ali DW, Wang Q, Tanowitz M, Du QS, et al. Regulation of neuregulin signaling by PSD-95 interacting with ErbB4 at CNS synapses. Neuron 2000; 26:443 - 455
- Bjarnadottir M, Misner DL, Haverfield-Gross S, Bruun S, Helgason VG, Stefansson H, et al. Neuregulin1 (NRG1) signaling through Fyn modulates NMDA receptor phosphorylation: differential synaptic function in NRG1+/− knock-outs compared with wild-type mice. J Neurosci 2007; 27:4519 - 4529
- Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet 2002; 71:877 - 892
- Seeman P, Schwarz J, Chen JF, Szechtman H, Perreault M, McKnight GS, et al. Psychosis pathways converge via D2(High) dopamine receptors. Synapse 2006; 60:319 - 346
- Horacek J, Bubenikova-Valesova V, Kopecek M, Palenicek T, Dockery C, Mohr P, et al. Mechanism of action of atypical antipsychotic drugs and the neurobiology of schizophrenia. CNS Drugs 2006; 20:389 - 409
- Kwon OB, Paredes D, Gonzalez CM, Neddens J, Hernandez L, Vullhorst D, et al. Neuregulin-1 regulates LTP at CA1 hippocampal synapses through activation of dopamine D4 receptors. Proc Natl Acad Sci USA 2008; 105:15587 - 15592
- Gasbarri A, Sulli A, Packard MG. The dopaminergic mesencephalic projections to the hippocampal formation in the rat. Prog Neuropsychopharmacol Biol Psychiatry 1997; 21:1 - 22
- Li S, Cullen WK, Anwyl R, Rowan MJ. Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nat Neurosci 2003; 6:526 - 531
- Huang YY, Kandel ER. D1/D5 receptor agonists induce a protein synthesis-dependent late potentiation in the CA1 region of the hippocampus. Proc Natl Acad Sci USA 1995; 92:2446 - 2450
- Granado N, Ortiz O, Suarez LM, Martin ED, Cena V, Solis JM, et al. D1 but not D5 dopamine receptors are critical for LTP, spatial learning, and LTP-Induced arc and zif268 expression in the hippocampus. Cereb Cortex 2008; 18:1 - 12
- Lemon N, Manahan-Vaughan D. Dopamine D1/D5 receptors gate the acquisition of novel information through hippocampal long-term potentiation and long-term depression. J Neurosci 2006; 26:7723 - 7729
- Williams S, Mmbaga N, Chirwa S. Dopaminergic D1 receptor agonist SKF 38393 induces GAP-43 expression and long-term potentiation in hippocampus in vivo. Neurosci Lett 2006; 402:46 - 50
- Frey U, Schroeder H, Matthies H. Dopaminergic antagonists prevent long-term maintenance of posttetanic LTP in the CA1 region of rat hippocampal slices. Brain Res 1990; 522:69 - 75
- Frey U, Hartmann S, Matthies H. Domperidone, an inhibitor of the D2-receptor, blocks a late phase of an electrically induced long-term potentiation in the CA1-region in rats. Biomed Biochim Acta 1989; 48:473 - 476
- Mrzljak L, Bergson C, Pappy M, Huff R, Levenson R, Goldman-Rakic PS. Localization of dopamine D4 receptors in GABAergic neurons of the primate brain. Nature 1996; 381:245 - 248
- Noain D, Avale ME, Wedemeyer C, Calvo D, Peper M, Rubinstein M. Identification of brain neurons expressing the dopamine D4 receptor gene using BAC transgenic mice. Eur J Neurosci 2006; 24:2429 - 2438
- Khan ZU, Gutierrez A, Martin R, Penafiel A, Rivera A, De La Calle A. Differential regional and cellular distribution of dopamine D2-like receptors: an immunocytochemical study of subtype-specific antibodies in rat and human brain. J Comp Neurol 1998; 402:353 - 371
- Gasbarri A, Verney C, Innocenzi R, Campana E, Pacitti C. Mesolimbic dopaminergic neurons innervating the hippocampal formation in the rat: a combined retrograde tracing and immunohistochemical study. Brain Res 1994; 668:71 - 79
- Jansson A, Goldstein M, Tinner B, Zoli M, Meador-Woodruff JH, Lew JY, et al. On the distribution patterns of D1, D2, tyrosine hydroxylase and dopamine transporter immunoreactivities in the ventral striatum of the rat. Neuroscience 1999; 89:473 - 489
- Woo RS, Li XM, Tao Y, Carpenter-Hyland E, Huang YZ, Weber J, et al. Neuregulin-1 Enhances Depolarization-Induced GABA Release. Neuron 2007; 54:599 - 610
- Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology 2001; 25:1 - 27
- Blum BP, Mann JJ. The GABAergic system in schizophrenia. Int J Neuropsychopharmacol 2002; 5:159 - 179
- Coyle JT. The GABA-glutamate connection in schizophrenia: which is the proximate cause?. Biochem Pharmacol 2004; 68:1507 - 1514
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci 2005; 6:312 - 324
- Fisahn A, Neddens J, Yan L, Buonanno A. Neuregulin-1 Modulates Hippocampal Gamma Oscillations: Implications for Schizophrenia. Cereb Cortex 2008; http://dx.doi.org/10.1093/cercor/bhn107
- Fox IJ, Kornblum HI. Developmental profile of ErbB receptors in murine central nervous system: implications for functional interactions. J Neurosci Res 2005; 79:584 - 597
- Yau HJ, Wang HF, Lai C, Liu FC. Neural development of the neuregulin receptor ErbB4 in the cerebral cortex and the hippocampus: preferential expression by interneurons tangentially migrating from the ganglionic eminences. Cereb Cortex 2003; 13:252 - 264
- Corfas G, Rosen KM, Aratake H, Krauss R, Fischbach GD. Differential expression of ARIA isoforms in the rat brain. Neuron 1995; 14:103 - 115
- Eilam R, Pinkas-Kramarski R, Ratzkin BJ, Segal M, Yarden Y. Activity-dependent regulation of Neu differentiation factor/neuregulin expression in rat brain. Proc Natl Acad Sci USA 1998; 95:1888 - 1893
- Pinkas-Kramarski R, Eilam R, Spiegler O, Lavi S, Liu N, Chang D, et al. Brain neurons and glial cells express Neu differentiation factor/heregulin: a survival factor for astrocytes. Proc Natl Acad Sci USA 1994; 91:9387 - 9391
- Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci 2008; 31:234 - 242
- Anton ES, Ghashghaei HT, Weber JL, McCann C, Fischer TM, Cheung ID, et al. Receptor tyrosine kinase ErbB4 modulates neuroblast migration and placement in the adult forebrain. Nat Neurosci 2004; 7:1319 - 1328
- Flames N, Long JE, Garratt AN, Fischer TM, Gassmann M, Birchmeier C, et al. Short- and long-range attraction of cortical GABAergic interneurons by neuregulin-1. Neuron 2004; 44:251 - 261
- Ghashghaei HT, Weber J, Pevny L, Schmid R, Schwab MH, Lloyd KC, et al. The role of neuregulin-ErbB4 interactions on the proliferation and organization of cells in the subventricular zone. Proc Natl Acad Sci USA 2006; 103:1930 - 1935
- Krivosheya D, Tapia L, Levinson JN, Huang K, Kang Y, Hines R, et al. ErbB4-Neuregulin signaling modulates synapse development and dendritic arborization through distinct mechanisms. J Biol Chem 2008; 283:32944 - 32956
- Li B, Woo RS, Mei L, Malinow R. The Neuregulin-1 Receptor ErbB4 Controls Glutamatergic Synapse Maturation and Plasticity. Neuron 2007; 54:583 - 597
- Corfas G, Roy K, Buxbaum JD. Neuregulin 1-erbB signaling and the molecular/cellular basis of schizophrenia. Nat Neurosci 2004; 7:575 - 580
- Roy K, Murtie JC, El-Khodor BF, Edgar N, Sardi SP, Hooks BM, et al. Loss of erbB signaling in oligodendrocytes alters myelin and dopaminergic function, a potential mechanism for neuropsychiatric disorders. Proc Natl Acad Sci USA 2007; 104:8131 - 8136
- Brinkmann BG, Agarwal A, Sereda MW, Garratt AN, Muller T, Wende H, et al. Neuregulin-1/ErbB signaling serves distinct functions in myelination of the peripheral and central nervous system. Neuron 2008; 59:581 - 595
- Hall J, Whalley HC, Job DE, Baig BJ, McIntosh AM, Evans KL, et al. A neuregulin 1 variant associated with abnormal cortical function and psychotic symptoms. Nat Neurosci 2006; 9:1477 - 1478
- Keri S, Kiss I, Kelemen O. Effects of a neuregulin 1 variant on conversion to schizophrenia and schizophreniform disorder in people at high risk for psychosis. Mol Psychiatry 2009; 14:118 - 119
- Crowley JJ, Keefe RS, Perkins DO, Stroup TS, Lieberman JA, Sullivan PF. The neuregulin 1 promoter polymorphism rs6994992 is not associated with chronic schizophrenia or neurocognition. Am J Med Genet B Neuropsychiatr Genet 2008; 147B:1298 - 1300
- Law AJ, Lipska BK, Weickert CS, Hyde TM, Straub RE, Hashimoto R, et al. Neuregulin 1 transcripts are differentially expressed in schizophrenia and regulated by 5′ SNPs associated with the disease. Proc Natl Acad Sci USA 2006; 103:6747 - 6752
- Silberberg G, Darvasi A, Pinkas-Kramarski R, Navon R. The involvement of ErbB4 with schizophrenia: association and expression studies. Am J Med Genet B Neuropsychiatr Genet 2006; 141:142 - 148
- Hahn CG, Wang HY, Cho DS, Talbot K, Gur RE, Berrettini WH, et al. Altered neuregulin 1-erbB4 signaling contributes to NMDA> receptor hypofunction in schizophrenia. Nat Med 2006; 12:824 - 828
- Chang Q, Fischbach GD. An acute effect of neuregulin 1 β to suppress α7-containing nicotinic acetylcholine receptors in hippocampal interneurons. J Neurosci 2006; 26:11295 - 11303
- Hancock ML, Canetta SE, Role LW, Talmage DA. Presynaptic type III neuregulin1-ErbB signaling targets α7 nicotinic acetylcholine receptors to axons. J Cell Biol 2008; 181:511 - 521
- Zhong C, Du C, Hancock M, Mertz M, Talmage DA, Role LW. Presynaptic type III neuregulin 1 is required for sustained enhancement of hippocampal transmission by nicotine and for axonal targeting of α7 nicotinic acetylcholine receptors. J Neurosci 2008; 28:9111 - 9116
- Martin LF, Freedman R. Schizophrenia and the α7 Nicotinic Acetylcholine Receptor. Int Rev Neurobiol 2007; 78:225 - 246
- Severance EG, Yolken RH. Novel α7 nicotinic receptor isoforms and deficient cholinergic transcription in schizophrenia. Genes Brain Behav 2008; 7:37 - 45
- Longart M, Liu Y, Karavanova I, Buonanno A. Neuregulin-2 is developmentally regulated and targeted to dendrites of central neurons. J Comp Neurol 2004; 472:156 - 172
- Britto JM, Lukehurst S, Weller R, Fraser C, Qiu Y, Hertzog P, et al. Generation and characterization of neuregulin-2-deficient mice. Mol Cell Biol 2004; 24:8221 - 8226