Abstract
Efficient assembly of a mitotic spindle and stable attachment of microtubules (k-fibers) to kinetochores are essential for the high fidelity of chromosome segregation. Both spindle assembly and Mitotic spindle mediates the segregation of chromosomes in the cell cycle and the proper function of the spindle is crucial to the high fidelity of chromosome segregation and to the stability of the genome. Nucleation of microtubules (MTs) from centrosomes and chromatin represents two well-characterized pathways essential for the assembly of a dynamic spindle in mitosis. Recently, we identified a third MT nucleation pathway, in which existing MTs in the spindle acts as a template to promote the nucleation and polymerization of MTs, thereby efficiently amplifying MTs in the spindle. We will review here our current understanding on the molecular mechanism, the physiological function and the cell-cycle regulation of MT amplification.k-fiber formation require robust nucleation and polymerization of microtubules mediated by the γ-tubulin ring complex (γ TuRC). It has been well established that centrosomes and chromatin are the two centers for microtubule nucleation. We recently demonstrate a third mechanism for microtubule nucleation and polymerization, in which the existing microtubules in the spindle act as templates to promote the formation of new microtubules. We showed that a novel spindle-associated protein, FAM29A, plays a critical role in this microtubule-dependent microtubule amplification. FAM29A associates with spindle microtubules and directly interacts with and recruits NEDD1, the targeting subunit of γTuRC. Spindle-associated γTuRC then promotes microtubule nucleation required for spindle assembly and k-fiber formation. This novel microtubule amplification pathway provides a powerful mechanism to control the local cytoskeleton structures independent of centrosomes and chromatin. We speculate that microtubule amplification not only functions in mitosis, but may also act in other physiological processes to re-enforce existing cytoskeleton structures.
Nucleation of microtubules (MTs) by the γ-tubulin ring complex (γ-TuRC) is the first step in the formation of the mitotic spindle.Citation1 MT nucleation and polymerization is also required for the maintenance of the spindle structure. Loss-of-function in one of the γ-tubulin isoforms in mice generates highly disorganized spindle and abnormal spindle poles, resulting in a characteristic mitotic arrest.Citation2 Similarly, knockdown of NEDD1, an accessory subunit of the γ-TuRC that targets γ-tubulin to various mitotic structures,Citation3,Citation4 reduces the density of spindle MTs and leads to a stable prometaphase arrest.Citation4 Thus, efficient nucleation and proper regulation of MTs are essential for normal mitotic progression and for the high fidelity of chromosome segregation in mitosis.
γ-TuRC is localized to centrosomes, the main microtubule organizing center in eukaryotic cells.Citation1,Citation5 NEDD1 recruits γ-TuRC to centrosomes to initiate MT nucleation and polymerization.Citation3,Citation4 Although centrosomes increase the efficiency of MT nucleation, they are not required for mitosis, as a spindle can form in the absence of functional centrosomes.Citation6 In mitosis, chromatin also promotes the polymerization of MTs through its associated guanine nucleotide exchange factor, RCC1, which generates a gradient of RanGTP around chromatin.Citation7 The RanGTP gradient dissociates the spindle assembly factors (SAFs) from their interactions with inhibitory importins and free SAFs then promote MT growth around the chromatin.Citation8–Citation10 Lastly, both NEDD1 and γ-tubulin are localized to the mitotic spindle,Citation4 suggesting that MTs may nucleate and polymerize from existing MTs in the spindle. We recently demonstrate that a novel spindle-associated protein, FAM29A, is required for targeting NEDD1 and γ-tubulin to the spindle and that FAM29A promotes the MT-dependent MT polymerization critical for the assembly of mitotic spindle and essential for the maturation of kinetochore MT fibers (k-fibers).Citation11
We initially identified FAM29A as a protein interacting with the Polo-like kinase, Plk1, an essential mitotic kinase that controls spindle assembly and its bipolarity.Citation12,Citation13 We found that FAM29A is a MT-associated protein (MAP) and that its MAP activity is regulated in the cell cycle.Citation11 It associates with MTs in vitro in the presence of mitotic extracts and colocalizes with MTs in vivo, but only during mitosis. In fact, in prometaphase and metaphase cells, FAM29A preferentially associates with cold-resistant stable k-fibers. FAM29A directly interacts with Plk1 and the active kinase of Plk1 targets FAM29A to the mitotic spindle. FAM29A also interacts with NEDD1, again only in mitosis. Through this direct interaction, spindle-associated FAM29A targets NEDD1 to the spindle and promotes the MT nucleation through NEDD1-associated γ-TuRC. Thus, FAM29A uses existing spindle MTs as templates to generate addition MTs, a phenomena we termed “MT amplification” ().
MT amplification is important to proper spindle assembly.Citation11,Citation14 Knockdown of FAM29A prevents the association of NEDD1 and γ-tubulin with the spindle and reduces the MT density by 60%.Citation11 The weak bipolar spindle remained in the FAM29A-depleted cells is not sufficient to efficiently capture the chromosomes and there is a delay in the progression through prometaphase. In mitotic cells that are released from an arrest by nocodazole, a MT destabilizing drug, the initial kinetics of MTs nucleation and polymerization from centrosomes and chromatin is independent of FAM29A. However, once the initial seeds of MTs are formed, the subsequent expansion of MTs is absent and the bipolar spindle fails to form in FAM29A-depleted cells. Thus, MT amplification provides sufficient amounts of MTs critical for spindle assembly.
MT amplification is also essential for the maturation of k-fibers.Citation11 Upon entry into mitosis, individual MTs extend from centrosomes, search the three-dimensional cytoplasmic space for chromosomes, and capture the kinetochores. The dynamic turnover of kinetochoreattached MTs provides the driving force for chromosome congression and segregation.Citation15,Citation16 However, a single attached MT is not sufficient to drive the chromosome movement and a mature k-fiber usually consists of 25–30 MTs that act in concert.Citation17 How a single attached MT quickly matures into a bundle of 25–30 MTs is a central mystery in cell biology. We showed that FAM29A-mediated MT amplification is essential for the maturation of k-fibers.Citation11 Depletion of FAM29A reduces the number of MTs per k-fiber, destabilizes the k-fibers, weakens the MT-kinetochore attachment, activates the spindle assembly checkpoint, and generates a profound metaphase arrest. We propose that in prometaphase cells, once a MT is attached to a kinetochore, and therefore is stabilized, the FAM29A-NEDD1 complex associated with this MT can quickly generate additional MTs using existing MT as a template. Subsequent bundling and poleward flux of MTs lead to a stable k-fiber that congresses the chromosome ().
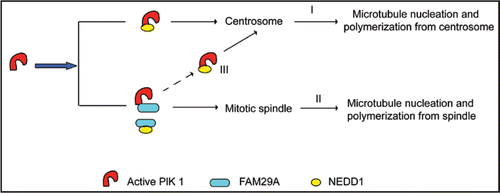
NEDD1 and γ-tubulin are localized to centrosomes and to the spindle and MTs nucleate from both of these mitotic structures.Citation4 How is MT nucleation from the spindle coordinated with MT nucleation from centrosomes? We found that recruitment of NEDD1 to both centrosomes and the spindle requires the kinase activity of Plk1. On the other hand, FAM29A is only required for targeting NEDD1 to the spindle, not to centrosomes () Citation11. Biochemically, Plk1 directly interacts with NEDD1, an interaction independent of FAM29A. This interaction is responsible for Plk1-mediated recruiting NEDD1 to centrosomes (, pathway I), but does not contribute to its localization to the spindle. On the other hand, recruitment of NEDD1 to the spindle depends on the interaction between Plk1 and FAM29A as well as the interaction between FAM29A and NEDD1 (, pathway II). Interestingly, reducing the levels of the FAM29A protein decreases the NEDD1 signals on the spindle, but increases its signals on centrosomes, indicating that the levels of FAM29A determine the partition of NEDD1 between centrosomes and the spindle, which, in turn, determine the relative contributions of MT nucleation and polymerization between these two mitotic structures (, pathway III).
FAM29A-mediated MT amplification is an evolutionarily conserved mechanism. For example, the Augmin complex recently identified in Drosophila has a subunit that shares a weak sequence homology to FAM29A and processes a similar biological activity.Citation14 Furthermore, MT amplification is not unique to mitosis. Plant cells lack a MT organizing center, and γ-tubulin is associated with cortical MTs to promote the polymerization of MTs that branch from existing cortical MTs.Citation18 Similarly, γ-tubulin in yeast is associated with interphase MTs and promotes the generation of new MTs to form MT bundles.Citation19 We speculate that MT amplification also acts beyond the cell cycle in physiological processes in mammalian cells, such as in neurons, where MT amplification promotes the local control of cytoskeleton structure independent of centrosomes.
Figures and Tables
Figure 1 FAM29A mediates MT amplification in spindle assembly and in k-fiber maturation. Active kinase of Plk1 targets FAM29A to spindle microtubules and FAM29A directly interacts with and recruits NEDD1, the targeting subunit of γTuRC. Spindle-associated γTuRC then promotes microtubule nucleation required for spindle assembly and k-fiber formation. Drawings for various cellular structures are representative, but not to the scale.
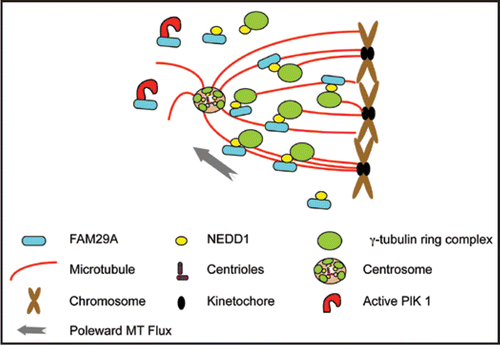
Figure 2 FAM29A controls the partition of NEDD1 between spindle MTs and centrosomes. The interaction between Plk1 and NEDD1 is responsible for Plk1-mediated recruitment of NEDD1 to centrosomes, which controls microtubule nucleation and polymerization from centrosomes (I). Recruitment of NEDD1 to the spindle depends on the interaction between Plk1 and FAM29A as well as the interaction between FAM29A and NEDD1, which mediate microtubule nucleation and polymerization from the mitotic spindle (II). The levels of FAM29A determine the partition of NEDD1 between centrosomes and the spindle, which, in turn, controls the relative contributions of MT nucleation and polymerization between these two mitotic structures (III).
Addendum to:
References
- Wiese C, Zheng Y. Microtubule nucleation: gamma-tubulin and beyond. J Cell Sci 2006; 119:4143 - 4153
- Yuba-Kubo A, Kubo A, Hata M, Tsukita S. Gene knockout analysis of two gamma-tubulin isoforms in mice. Dev Biol 2005; 282:361 - 373
- Haren L, Remy MH, Bazin I, Callebaut I, Wright M, Merdes A. NEDD1-dependent recruitment of the gamma-tubulin ring complex to the centrosome is necessary for centriole duplication and spindle assembly. J Cell Biol 2006; 172:505 - 515
- Luders J, Patel UK, Stearns T. GCP-WD is a gamma-tubulin targeting factor required for centrosomal and chromatin-mediated microtubule nucleation. Nat Cell Biol 2006; 8:137 - 147
- Luders J, Stearns T. Microtubule-organizing centres: a re-evaluation. Nat Rev Mol Cell Biol 2007; 8:161 - 167
- Mahoney NM, Goshima G, Douglass AD, Vale RD. Making microtubules and mitotic spindles in cells without functional centrosomes. Curr Biol 2006; 16:564 - 569
- Bastiaens P, Caudron M, Niethammer P, Karsenti E. Gradients in the self-organization of the mitotic spindle. Trends Cell Biol 2006; 16:125 - 134
- Gruss OJ, Vernos I. The mechanism of spindle assembly: functions of Ran and its target TPX2. J Cell Biol 2004; 166:949 - 955
- Nachury MV, Maresca TJ, Salmon WC, Waterman-Storer CM, Heald R, Weis K. Importin beta is a mitotic target of the small GTPase Ran in spindle assembly. Cell 2001; 104:95 - 106
- Karsenti E, Vernos I. The mitotic spindle: a self-made machine. Science 2001; 294:543 - 547
- Zhu H, Coppinger JA, Jang CY, Yates JR 3rd, Fang G. FAM29A promotes microtubule amplification via recruitment of the NEDD1-gamma-tubulin complex to the mitotic spindle. J Cell Biol 2008; 183:835 - 848
- van Vugt MA, van de Weerdt BC, Vader G, Janssen H, Calafat J, Klompmaker R, et al. Polo-like kinase-1 is required for bipolar spindle formation but is dispensable for anaphase promoting complex/Cdc20 activation and initiation of cytokinesis. J Biol Chem 2004; 279:36841 - 36854
- Barr FA, Sillje HH, Nigg EA. Polo-like kinases and the orchestration of cell division. Nat Rev Mol Cell Biol 2004; 5:429 - 440
- Goshima G, Mayer M, Zhang N, Stuurman N, Vale RD. Augmin: a protein complex required for centrosome-independent microtubule generation within the spindle. J Cell Biol 2008; 181:421 - 429
- Maiato H, DeLuca J, Salmon ED, Earnshaw WC. The dynamic kinetochore-microtubule interface. J Cell Sci 2004; 117:5461 - 5477
- Kirschner M, Mitchison T. Beyond self-assembly: from microtubules to morphogenesis. Cell 1986; 45:329 - 342
- McIntosh JR, Grishchuk EL, West RR. Chromosome-microtubule interactions during mitosis. Annu Rev Cell Dev Biol 2002; 18:193 - 219
- Murata T, Sonobe S, Baskin TI, Hyodo S, Hasezawa S, Nagata T, et al. Microtubule-dependent microtubule nucleation based on recruitment of gamma-tubulin in higher plants. Nat Cell Biol 2005; 7:961 - 968
- Janson ME, Setty TG, Paoletti A, Tran PT. Efficient formation of bipolar microtubule bundles requires microtubule-bound gamma-tubulin complexes. J Cell Biol 2005; 169:297 - 308