Abstract
The bicyclic nitroimidazole PA-824 is a pro-drug with a very complex mechanism of action active against both replicating and hypoxic, non-replicating Mycobacterium tuberculosis. Microarray analysis of the mode of action of PA-824 showed a puzzling mixed effect both on genes responsive to both cell wall inhibition (like isoniazid) and respiratory poisoning (like cyanide). The aerobic killing mechanism of this drug appears to involve inhibition of cell wall mycolic acid biosynthesis through an as yet unknown molecular mechanism. However, the structure-activity relationships governing aerobic activity do not parallel the relationships determining anaerobic activity. Based on the metabolite profiling of PA-824 and various derivatives by Ddn-mediated activation, we have shown that PA-824 acts directly as an NO donor.1 This respiratory poisoning through nitric oxide release seemed to be a crucial element of anaerobic activity by PA-824. The effect of PA-824 on the respiratory complex under hypoxic non-replicating conditions was also manifested in a rapid drop in intracellular ATP levels, again similar to that observed by cyanide treatment. Thus, transcriptional profiling provided valuable clues to elucidating the molecular mechanism of mycobacterial killing.
Mycobacterium tuberculosis (Mtb), the causative agent of tuberculosis (TB), has an amazing ability to persist in the human host.Citation2 The current TB drugs are highly effective against actively replicating bacilli and largely ineffective against persistent forms, leading to a current interest in developing new TB drugs which target persistent bacilli.Citation3 Bicyclic nitroimidazoles like PA-824 and OPC-67683 are an interesting class of anti-tuberculosis compounds that have inhibitory activity against both actively replicating and hypoxic non-replicating Mtb.Citation4,Citation5 Both compounds are pro-drugs activated by a deazaflavin (cofactor F420) dependent nitroreducase (Ddn).Citation6,Citation7 Treatment of aerobically-replicating cells with PA-824 rapidly disrupts the formation of ketomycolates with concomitant accumulation of hydroxymycolates,Citation8 a class of mycolic acids that are major constituents of the cell envelope of Mtb. OPC-67683, a related bicyclic nitroimidazole, was optimized chemically based upon its ability to inhibit mycolic acid synthesis.Citation7 However, while there is obviously a connection with cell-wall inhibition under aerobic conditions, this effect seemed unlikely to be responsible for cell killing under non-replicating conditions since the bacilli do not extensively remodel mycolic acids under anaerobiosis.Citation9 Further, no other known mycolic acid synthesis inhibitors kill Mtb cells under hypoxic non-replicating conditions.
To gain further insight into the mechanism of action, we examined the transcriptional profiles of Mtb treated with PA-824 under aerobic conditions and compared them with transcriptional profiles of 430 inhibitors of mycobacterial metabolism.Citation10 These studies revealed that PA-824 had a complex mode of action with significant effects on transcription of genes responsive to known inhibitors of cell wall synthesis (such as isoniazid, thiolactomycin, ethionamide and cerulenin) as well as on genes responsive to respiratory poisons (such as potassium cyanide) (). Unfortunately, such transcriptional profiling can not be done with hypoxic cells because they have extremely low basal transcription rates. Ultimately this data suggested that the drug has two discrete targets, one from each class. To confirm this, expression of a few highly responsive genes with PA-824 treatment under aerobic conditions was confirmed by the quantitative reverse transcription-PCR analysis (). PA-824 treatment resulted in an upregulation of the fasI gene as well as many genes in the fasII operon; along with efpA and iniBAC operon. Several cell wall biosynthesis inhibitors have been shown to upregulate iniBAC operon.Citation10,Citation11
Unlike other mycolic acid synthesis inhibitors, PA-824 treatment also resulted in an upregulation of the cyd operon (cydA, cydB, cydD and cydC) encoding the non-proton-pumping cytochrome bd oxidase, the nitrate reductase narGHIJ and other genes involved in respiration. This transcriptional profile was very similar to cytochrome c oxidase-specific inhibitors like potassium cyanide (). Respiratory inhibitors, such as potassium cyanide, rapidly change the redox status of the cells, an effect that can be measured by examining the quinol/quinone pool. Like cyanide, PA-824 dramatically shifted the predominant isoprenoid quinol/quinone ratio (MK9H2/MK9) in a time and concentration dependent manner (). The effect of PA-824 on the respiratory complex under hypoxic non-replicating conditions was also manifested by a rapid drop in intracellular ATP levels (). This drop occurred within 2 hours of compound addition, whereas aerobically replicating cells treated with PA-824 did not show a drop in ATP within the first 24 hours of treatment (results not shown).
These observations led us to believe that the mechanism of action of PA-824 involved the inhibition of a fundamental component of energy metabolism and also encouraged us to look into the mechanism of Ddn-mediated bioreduction of these compounds in more detail. Based on metabolite profiling of PA-824 and its analogues by Ddn, we have recently shown that these bicyclic nitroimidazoles act as [NO] donors, and that NO release from various PA-824 derivatives correlated well with the anaerobic killing of Mtb.Citation1 Respiratory poisoning through nitric oxide release seems to be the crucial element of the anaerobic activity by PA-824. Thus PA-824 acts as a “suicide bomb” releasing toxic NO within mycobacterial cells and NO possibly reacts with cytochromes/cytochrome oxidase to interfere with the electron flow and ATP homeostasis under hypoxic non-replicating conditions. This NO-releasing effect of PA-824 is not sufficient to kill aerobically replicating cells. Likewise cyanide has little effect on aerobically replicating Mtb. Maintenance of an energised membrane and ATP homeostasis have been shown to be significant points of metabolic vulnerability in hypoxic non-replicating mycobacteria.Citation12,Citation13
In Mycobacterium, NO is known to have multiple targets including DNA,Citation14 as many as 29 mycobacterial enzymes,Citation15 including the ATP synthase, Pks13, RpoB and recently has been shown to displace copper in a toxic form from a metallothionein.Citation16 However, under aerobic conditions even though NO is released in mycobacterial cells,Citation1 it doesn't seem to play a critical role in cell killing. It is likely because the inhibition of cytochrome c oxidase by NO is reversible in the presence of oxygen.Citation17,Citation18
Thus, transcriptional profiling of PA-824 provided a fundamental insight into the involvement of respiratory poisoning and was a vital clue to elucidating the anaerobic mechanism of action. Of course, predictions based on transcriptional profiling need supporting evidence and often they merely generate hypotheses for further work. In the case of PA-824, the microarray data primed us to consider chemically plausible ways to generate a non-specific toxic metabolite.
Figures and Tables
Figure 1 Transcriptional response profiles of M. tuberculosis to PA-824 and known respiratory and fatty acid synthesis inhibitors. Heat map rendered table of gene expression changes for those genes that predictively associate PA-824 with either KCN or fatty acid synthesis inhibitors as described earlier.Citation10 Inset shows color scale for expression ratios.
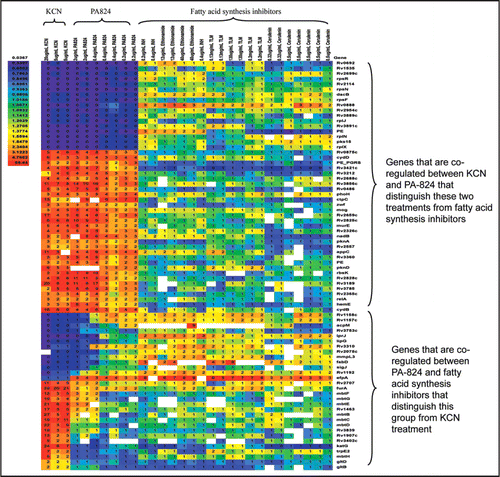
Figure 2 qRT-PCR analysis of selected genes with PA-824 treatment. Early mid-log phase cells treated with PA-824 2 µg/ml for 2 hrs prior to RNA extraction and qRT-PCR performed by Taqman analysis as described.Citation10 Expression levels of genes normalized to levels of the sigA mRNA.
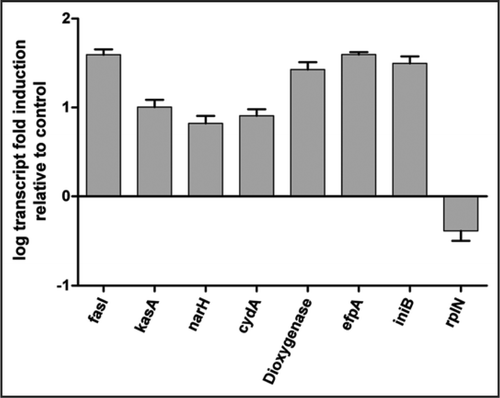
Figure 3 Effect of PA-824 on the redox status of menaquinol/menaquinone (MK9H2/MK9). Early mid-log phase cells were treated with PA-824 for 1 hr (A) or as indicated (B) before cells were pelletted for menaquinone analysis as described.Citation10 Shown is the typical result of one of two independent experiments.
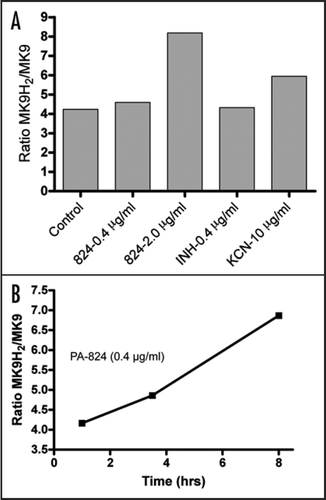
Figure 4 Effect of PA-824 treatment on cellular ATP levels on NRP-2 cells. Intracellular ATP was quantified by using the BacTiter-Glo Microbial Cell Viability Assay Kit (Promega). Anaerobically adapted cells were treated in white 96-well plates (100 µl/well) with different concentrations of PA-824 or DMSO alone at a final DMSO concentration of 0.2%. At 0, 2 and 4 hours, plates were removed from the anaerobic chamber and 20 µl of BacTiter-Glo reagent to each well. Luminescence was recorded 10 min after incubation in a Fluostar Optima plate reader (BMG Labtech).
Acknowledgements
This work was funded (in part) by the intramural research program of National Institute of Allergy and Infectious Diseases, NIH, and (in part) by a grant from the Bill and Melinda Gates Foundation and the Wellcome Trust through the Grand Challenges in Global Health Initiative.
Addendum to:
References
- Singh R, Manjunatha U, Boshoff HI, Ha YH, Niyomrattanakit P, Ledwidge R, et al. PA-824 kills nonreplicating Mycobacterium tuberculosis by intracellular NO release. Science 2008; 322:1392 - 1395
- Matteelli A, Migliori GB, Cirillo D, Centis R, Girard E, Raviglion M. Multidrug-resistant and extensively drug-resistant Mycobacterium tuberculosis: epidemiology and control. Expert Rev Anti Infect Ther 2007; 5:857 - 871
- Sacchettini JC, Rubin EJ, Freundlich JS. Drugs versus bugs: in pursuit of the persistent predator Mycobacterium tuberculosis. Nat Rev Microbiol 2008; 6:41 - 52
- Barry CE 3rd, Boshoff HI, Dowd CS. Prospects for clinical introduction of nitroimidazole antibiotics for the treatment of tuberculosis. Curr Pharm Des 2004; 10:3239 - 3262
- StopTB. StopTB Working Group on New Drugs 2008; www.stoptb.org/wg/new_drugs/assets/documents/2007GlobalPipeline.pdf
- Manjunatha UH, Boshoff H, Dowd CS, Zhang L, Albert TJ, Norton JE, et al. Identification of a nitroimidazo-oxazine-specific protein involved in PA-824 resistance in Mycobacterium tuberculosis. Proc Natl Acad Sci USA 2006; 103:431 - 436
- Matsumoto M, Hashizume H, Tomishige T, Kawasaki M, Tsubouchi H, Sasaki H, et al. OPC-67683, a nitro-dihydro-imidazooxazole derivative with promising action against tuberculosis in vitro and in mice. PLoS Med 2006; 3:466
- Stover CK, Warrener P, VanDevanter DR, Sherman DR, Arain TM, Langhorne MH, et al. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature 2000; 405:962 - 966
- Boshoff HI, Barry CE. Is the mycobacterial cell wall a hopeless drug target for latent tuberculosis?. Drug Disc Tod Dis Mech 2006; 3:237 - 245
- Boshoff HI, Myers TG, Copp BR, McNeil MR, Wilson MA, Barry CE 3rd. The transcriptional responses of Mycobacterium tuberculosis to inhibitors of metabolism: novel insights into drug mechanisms of action. J Biol Chem 2004; 279:40174 - 40184
- Wilson M, DeRisi J, Kristensen HH, Imboden P, Rane S, Brown PO, et al. Exploring drug-induced alterations in gene expression in Mycobacterium tuberculosis by microarray hybridization. Proc Natl Acad Sci USA 1999; 96:12833 - 12838
- Boshoff HI, Barry CE 3rd. Tuberculosis—metabolism and respiration in the absence of growth. Nat Rev Microbiol 2005; 3:70 - 80
- Rao SP, Alonso S, Rand L, Dick T, Pethe K. The protonmotive force is required for maintaining ATP homeostasis and viability of hypoxic, nonreplicating Mycobacterium tuberculosis. Proc Natl Acad Sci USA 2008; 105:11945 - 11950
- Darwin KH, Ehrt S, Gutierrez-Ramos JC, Weich N, Nathan CF. The proteasome of Mycobacterium tuberculosis is required for resistance to nitric oxide. Science 2003; 302:1963 - 1966
- Rhee KY, Erdjument-Bromage H, Tempst P, Nathan CF. S-nitroso proteome of Mycobacterium tuberculosis: Enzymes of intermediary metabolism and antioxidant defense. Proc Natl Acad Sci USA 2005; 102:467 - 472
- Gold B, Deng H, Bryk R, Vargas D, Eliezer D, Roberts J, et al. Identification of a copper-binding metallothionein in pathogenic mycobacteria. Nat Chem Biol 2008; 4:609 - 616
- Cleeter MW, Cooper JM, Darley-Usmar VM, Moncada S, Schapira AH. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett 1994; 345:50 - 54
- Brunori M, Forte E, Arese M, Mastronicola D, Giuffrè A, Sarti P. Nitric oxide and the respiratory enzyme. Biochim Biophys Acta 2006; 1757:1144 - 1154