Abstract
The bacterium Escherichia coli is rod-shaped, and a unit cell keeps regular dimensions of about 1.5 μm long and 0.5 μm wide. The rod-shaped cell is composed of two parts: a cylinder in the center and caps at both ends. The length of the cylinder corresponds to the length of the rod cell. A recent paper reported the genetic regulation of the cell length by rodZ. RodZ is a membrane protein with bitopic topology that assembles underneath the cell membrane to form helical filaments along the lateral axis of the cell with the bacterial actin MreB. RodZ filaments probably interact with enzymes that contribute to peptidoglycan synthesis. Cells lacking rodZ shorten only along the lateral axis of the cell so that the cells become round-shaped instead of rod-shaped. Such spheroidal cells consist only of caps due to the loss of almost all of the cylinder. In addition, carbon metabolism is remarkably disturbed by the deficiency of RodZ. This suggests that the transport of nutrients at the surface of the cylinder is reduced in rodZ mutant cells. Thus, cell morphology is also critical for proper metabolism for cell proliferation.
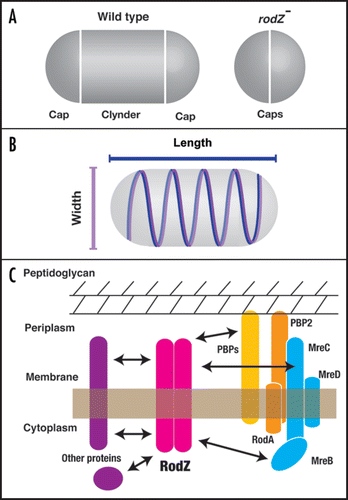
Cell shape is one of the critical features in bacterial species and is genetically determined. A group of bacterial cells including Escherichia coli and Bacillus subtilis look like a short stick, and are therefore called rods. The rod cell is separated into two parts (). The central region of the cell is a cylinder, and at both ends of the cylinder are caps. Before binary fission of the rod cell, the cylinder elongates to twice the size of a unit cell. Cell division occurs at the middle of the cylinder and the divided ends generate new caps. Since the rod cell is encompassed by a mechanically rigid cell wall, or peptidoglycan layer, degradation and synthesis of the peptidoglycan layer always accompanies cell division. The shape of the peptidoglycan layer is determined by cytoskeletal proteins underneath the cell membrane.
Cytoskeletal proteins help to maintain various cell shapes, except the simple round shape, even in prokaryotes.Citation1,Citation2 It has been shown that the bacterial actin MreB is responsible for maintenance of the rod-shape, with inactivation of MreB resulting in a change in cell shape from rod to round. MreB proteins form filaments in vivo and in vitro, and are widely conserved among rod-shaped bacteria.Citation3–Citation6 B. subtilis has two paralogs of mreB, and each gene product independently regulates the length and width of the cell.Citation3 However, many rod bacteria, including E. coli, have only one homolog of mreB. It had been a mystery as to how the cell size is regulated, but recently we found a second regulator of cell length in E. coli.Citation7,Citation8
The RodZ Filament as a Measure of Cell Length
A deletion mutant that causes cell shape to be round has been found in the gene knockout library of E. coli. Quantitative analysis of cell size indicates that the mutant gene is responsible for the maintenance of cell length. The mutant cell is shortened only along the long axis of the cell, not the short axis. In addition, RodZ fused with green fluorescent protein (GFP) shows helical filaments extending along the long axis of the cell underneath the cytoplasmic membrane. An excess of the RodZ protein causes the cell to elongate compared to the wild-type cell. It is unclear how RodZ determines the length of the long axis. An attractive hypothesis is that extension of RodZ filaments contributes to the regulation of cell length as a measure of size.
Interaction of RodZ with Other Factors
Formation of MreB filaments does not require the RodZ protein, and vice versa. Although RodZ filaments are formed independently of MreB filaments, they co-localize. In fact, Piet de Boer and colleagues have found that the two proteins interact in a bacterial two-hybrid assay.Citation8 As it is thought that MreB may regulate cell width, cooperative regulation of cell size would be performed by co-localization of MreB and RodZ ().
The RodZ protein is a membrane protein spanning the cyoplasmic membrane once, and is divided into cytoplasmic and periplasmic domains. Corresponding to this topology, the molecular function is separated. The cytoplasmic domain is required for formation of RodZ filaments. Interestingly, the filamentous structure is not necessary to keep the rod-shape. Nevertheless, RodZ helical filaments may be responsible for shaping an accurate rod cell. On the other hand, the transmembrane and periplasmic domains are responsible for rod-shape formation. The bitopic topology probably allows RodZ to interact with other proteins or complexes through the cytoplasmic and periplasmic domains. A reasonable idea is that the periplasmic domain of RodZ interacts with peptidoglycan synthesis enzymes such as PBPs in the periplasmic space (). Defects in enzymes involved in peptidoglycan synthesis produce cells with aberrant shapes. Similar shaped cells have been observed during restoration of rod-shapes from round-shapes upon resumption of production of the RodZ protein. Newly synthesized RodZ proteins might induce rearrangements of PBPs in the periplasmic space and then reconstruction of the peptidoglycan layer might take place.
Reduced Growth and Metabolic Perturbation
In addition to changing the cell shape, the growth rates of mutant cells are remarkably reduced. Surprisingly, the utilization of many carbon sources, except glucose, was dramatically downregulated as if the mutation occurred in metabolic genes.Citation9 Furthermore, ATP synthesis via glycolysis increases in mutant cells.Citation10 Glycolysis, which proceeds in the cytosol, is accelerated in the mutants instead of oxidative phosphorylation, which critically depends on the membrane. When the volume and a surface area of round cells are roughly calculated, the volume is increased by about 1.5-fold while the surface area is almost same as the wild type. The transport of nutrients might be impaired because of a decrease in the surface area to volume ratio. Alternatively, transporters at the surface of the cylinder used to incorporate nutrients may be reduced in rodZ mutant cells. Thus, the change of cell shape not only affects the structure of the cell membrane, it also affects various activities inside the cell for cell proliferation.
Figures and Tables
Figure 1 Role of RodZ in maintenance of the rod-shape in E. coli. (A) Wildtype rod-shaped E. coli consists of a lateral cylinder and polar caps. ΔrodZ cells lack the lateral cylinder and therefore consist only of polar caps. (B) RodZ (blue) and MreB (magenta) form regular helical filaments along the long axis of the cell in order to maintain the length of the long and short axes, respectively. (C) A model of an interaction network of RodZ and its related proteins. RodZ is a bitopic membrane protein with periplasmic and cytosolic domains. Each domain would interact with factors that are involved in peptidoglycan synthesis (PBPs; penicillin-binding proteins), the MreBCD complex, or other proteins that have not been identified.
Acknowledgements
This work was supported by the New Energy and Industrial Technology Development Organization (NEDO), and a Grant-in-Aid for Young Scientists (Start-up) and an NIG Postdoctoral Fellowship to Daisuke Shiomi.
Addendum to:
References
- Erickson HP. Evolution of the cytoskeleton. BioEssays 2007; 29:668 - 677
- Osborn MJ, Rothfield L. Cell shape determination in Escherichia coli. Curr Opin Microbiol 2007; 10:606 - 610
- Jones LJ, Carballido-Lopez R, Errington J. Control of cell shape in bacteria: helical, actin-like filaments in Bacillus subtilis. Cell 2001; 104:913 - 922
- Wachi M, Doi M, Okada Y, Matsuhashi M. New mre genes mreC and mreD, responsible for formation of the rod shape of Escherichia coli cells. J Bacteriol 1989; 171:6511 - 6516
- Shih YL, Le T, Rothfield L. Division site selection in Escherichia coli involves dynamic redistribution of Min proteins within coiled structures that extend between the two cell poles. Proc Natl Acad Sci USA 2003; 100:7865 - 7870
- van den Ent F, Amos LA, Lowe J. Prokaryotic origin of the actin cytoskeleton. Nature 2001; 413:39 - 44
- Shiomi D, Sakai M, Niki H. Determination of bacterial rod shape by a novel cytoskeletal membrane protein. EMBO J 2008; 27:3081 - 3091
- Bendezu FO, Hale CA, Bernhardt TG, de Boer PA. RodZ (YfgA) is required for proper assembly of the MreB actin cytoskeleton and cell shape in E. coli. EMBO J 2009; 28:193 - 204
- Ito M, Baba T, Mori H, Mori H. Functional analysis of 1,440 Escherichia coli genes using the combination of knock-out library and phenotype microarrays. Metab Eng 2005; 7:318 - 327
- Hara KY, Shimodate N, Ito M, Baba T, Mori H, Mori H. Systematic genome-wide scanning for genes involved in ATP generation in Escherichia coli. Metab Eng 2009; 11:1 - 7