Abstract
Heterotrimeric G proteins are key molecules regulating cellular responses to extracellular stimuli, and are composed of α, β and γ subunits. All α subunits in vertebrates belong to four major classes, Gs, Gi, Gq and G12, which are conserved throughout the animal kingdom. Unexpectedly, now a fifth class of Gα protein, Gv, has been discovered. Gv is conserved across the animal kingdom and present in vertebrates, arthropods, mollusks, annelids, and even sponges. Presumably, Gv has been missed so far, because it has been lost in many lineages in the major model organisms such as nematodes, fruit fly, and mammals. On the other hand, gene gains are also observed for Gv, with at least two independent gene duplications, one in sponges and the other in the teleost lineage. Such frequent gene gains and losses fit to a birth-and-death mode of evolution, which is unusual for a well-conserved and ancient gene family like the Gα proteins. The discovery of a novel major class of Gα proteins provides new insights in the evolution of the Gα protein family and opens new possibilities in G protein signaling research.
Heterotrimeric G proteins relay a broad range of extracellular signals received by G protein-coupled receptors (GPCRs) to many different intracellular signaling cascades.Citation1 Among the three subunits α, β and γ, the α subunits interact with GPCRs directly and act as a key switch by alternating between two guanine-nucleotide binding conformations: a GTP-binding active form and a GDP-binding inactive form.Citation2 Upon the receptor activation, Gα undergoes a conformational change coupled to GDP/GTP exchange and the heterotrimer dissociates into Gα and β/γ heterodimer. The GTP-binding active form signals to downstream pathways by regulating the activity of effector proteins such as adenylate cyclases and phospholipases. Therefore, Gα has a central role in the G protein signaling by converging a variety of inputs from GPCRs and allocating them to various downstream pathways. All vertebrate Gα proteins described so far belong to four major classes (Gs, Gi, Gq and G12) defined by their sequence homologies.Citation3,Citation4 Each class can be subdivided into two to four families; the Gs class contains Gαs and Gαolf; Gi comprises Gαt, Gαo, Gαi and Gαz; Gq encompasses Gαq, Gα11, Gα14 and Gα15/16; and G12 contains Gα12 and Gα13. Each class possesses particular sets of coupled receptors and target poteins although some overlap is observed. In contrast to the well-investigated mammalian, fruit fly and nematode Gα proteins, our knowledge about the Gα protein family in lower vertebrates and many invertebrate phyla is still very fragmentary.
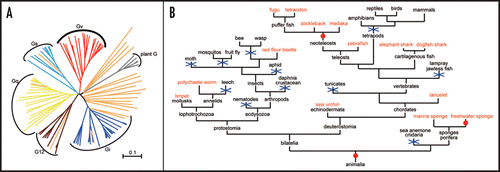
In light of the fact that teleost species are getting more and more importance as animal models for human health and disease, we recently analyzed the gna gene family in zebrafish and found a novel Gα protein that cannot be grouped into any of the four established classes, which we named Gv, for class V of Gα proteins.Citation5 Orthologous gnav genes are broadly distributed across the animal kingdom and representatives are found in sponges, vertebrates (teleosts and cartilaginous fish), cephalochordate (lancelet), echinoid (sea urchin), insect (red flour beetle), mollusk (limpet) and annelid (polychaete worm). When analyzed in phylogenetic trees, gnav genes did not cluster with the nematode-specific gpa genes (), which are the only previous exception for the canonical four classes in all of the animal kingdom.Citation6 All Gv proteins formed a single branch of the phylogenetic tree that constitutes a fifth class of metazoan Gα proteins, at the level of and as ancient as the other four classes (). Additional lines of evidence supported this assignment of Gv as a separate class in its own right, among them a distinct splicing pattern and many Gv-specific conserved sequence motifs. Thus it came as quite a shock that this class was lost in many lineages including tetrapods (mammals, chicken, reptiles and amphibians), jawless fish (lamprey), tunicates, many insects (fruit fly, mosquitoes, bee, wasp, moth and aphid), crustacean (daphnia), nematodes, annelid (leech) and cnidarian (sea anemone) (). On the other hand, we found at least two independent gene duplications giving rise to two paralogs in a sponge lineage and in neoteleosts (). Such a dynamic evolutionary behavior has been christened a birth-and-death mode of evolution,Citation7 and is unusual for a highly conserved gene family like the Gα proteins. However, the negative selective pressure as measured by the ratio of non-synonymous to synonymous nucleotide substitutions is somewhat relaxed for gnav genes compared to other Gα classes, suggesting a somewhat accelerated evolution of Gv. A more rapid accumulation of mutations may result more frequently in disfunctionalization and finally in gene loss.
What do we currently know about the function of Gv? As judged from sequence analysis, Gv proteins seem to retain all basic biochemical characteristics of Gα proteins.Citation2 All Gv proteins are predicted to have the domain structures typical for Gα proteins, the Ras-like GTPase domain and the helical domain. Furthermore, the switch regions, which undergo conformational change upon activation, the G-boxes, which form the GTP-binding pocket, and two amino acid residues critical for GTPase activity, as well as lipid modification sites at the N-terminal region, involved in membrane localization, are all properly arranged in the Gv molecules.
To fulfill their function, Gα proteins interact with several kinds of molecules: their upstream receptors (GPCRs), the other two G protein subunits (β/γ heterodimers), regulator and effector proteins. Each class or family of Gα has distinct interaction partners, although cross-talk is known to happen. Numerous modeling and mutational analyses reported critical residues for Gα interactions with partner molecules.Citation8 Gv proteins share some residues in switch regions which are thought to be involved in regulator/effector interactions. However, it is not straightforward to predict interactions of Gv with the molecules known to interact with other Gα proteins since the contact residues are not always conserved among orthologs, or even within a class or a family in a species. On the other side, a high number of Gv-specific motifs suggests the existence of Gv-specific GPCRs and regulator/effector proteins. Among these motifs are the class-specific sequence of both N- and C-terminal regions, which have been suggested to interact with GPCRs and β/γ heterodimers. Moreover, Gv proteins possess several stretches of specifically conserved residues in the helical domain, which also exhibits class or family-specific sequences and length. This domain is suggested to be involved in interactions with GPCRs, β/γ heterodimers and regulator/effector proteins.Citation9–Citation13 Taken together, Gv might modulate signaling pathways distinct from those of other classes.
The expression pattern of the zebrafish gnav1 gene suggests an involvement in sensory cell differentiation, as transcripts are found in the developing inner ear and an unidentified small cell population near the lip that could well be a primordium for barbels, i.e., taste buds. Expression in adult tissues is broader, but not ubiquitous and as such would be consistent with several different, yet to be identified functions.
Frequent gene loss in the evolution raises the possibility that in those species without Gv, subsets of GPCRs for Gv may have also been lost. As GPCRs are the largest gene family in most animal genomes and directly face the cellular and organismal environment, loss of Gv could affect the ecological adaptations of many animal species. It will be very interesting to see whether this idea will be corroborated after identification of GPCR molecules interacting with Gv and signaling pathways regulated through Gv will make such studies possible.
Can there be still another class of Gα proteins? The discovery of the fifth class of Gα proteins, Gv, nearly two decades after the fourth class was identified, makes you wonder about such a possibility. However, our search strove to identify all potential Gα proteins irrespective of their class assignment and covered several major branches of the tree of animal life. With the sole exception of nematodes none had Gα proteins outside the five classes. While we cannot exclude that lineage-specific Gα subfamilies like the nematode-specific gpa genes may surface in future genomic studies, such subfamilies are not likely to represent major subdivisions of the Gα family conserved across metazoan evolution. Thus we think that the fifth class, Gv, is probably the final class to be discovered in the animal kingdom.
Some open questions remain. The evolutionary position of animal Gα proteins relative to those in other kingdoms like plants and fungi is not completely clear, neither is the exact way of how the five classes have arisen from an ancestral Gα protein. Thanks to ever increasing efforts to build genome databases of primitive organisms, future studies will lead to a better understanding of the early evolution of metazoan Gα proteins. We would like to emphasize that it is important for any phylogenetic studies of a gene family to include species beyond the usual suspects (model organisms), as some subfamilies may escape one's notice due to their absence in the studied species.
Figures and Tables
Figure 1 Evolution of Gv. (A) The phylogenetic tree of Gα proteins. The class Gv (red) does not cluster with any of the other four classes (Gs, light blue; Gi, blue; Gq, yellow and G12, brown) collected from human, zebrafish, fruit fly, nor any Gα proteins of nematode (orange). Plant Gα proteins (grey) are included as outgroup. Scale bar indicates amino acid substitution rate. (B) Gene gain and loss events of Gv in animal evolution. Species in red contain at least one Gv ortholog; species in black, no Gv orthologs found; blue crosses, inferred gene loss event; red circle, gene gain event.
Addendum to:
References
- Wettschureck N, Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol Rev 2005; 85:1159 - 1204
- Oldham WM, Hamm HE. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat Rev Mol Cell Biol 2008; 9:60 - 71
- Strathmann MP, Simon MI. Gα12 and Gα13 subunits define a fourth class of G protein alpha subunits. Proc Natl Acad Sci USA 1991; 88:5582 - 5586
- Downes GB, Gautam N. The G protein subunit gene families. Genomics 1999; 62:544 - 552
- Oka Y, Saraiva LR, Kwan YY, Korsching SI. The fifth class of Galpha proteins. Proc Natl Acad Sci USA 2009; 106:1484 - 1489
- O'Halloran DM, Fitzpatrick DA, McCormack GP, McInerney JO, Burnell AM. The molecular phylogeny of a nematode-specific clade of heterotrimeric G-protein alpha-subunit genes. J Mol Evol 2006; 63:87 - 94
- Nei M, Rooney AP. Concerted and birth-and-death evolution of multigene families. Annu Rev Genet 2005; 39:121 - 152
- Sprang SR, Chen Z, Du X. Structural basis of effector regulation and signal termination in heterotrimeric Galpha proteins. Adv Protein Chem 2007; 74:1 - 65
- Krieger-Brauer HI, Medda PK, Hebling U, Kather H. An antibody directed against residues 100–119 within the alpha-helical domain of Galpha(s) defines a novel contact site for beta-adrenergic receptors. J Biol Chem 1999; 274:28308 - 28313
- Skiba NP, Yang CS, Huang T, Bae H, Hamm HE. The alpha-helical domain of Galphat determines specific interaction with regulator of G protein signaling 9. J Biol Chem 1999; 274:8770 - 8778
- Soundararajan M, Willard FS, Kimple AJ, Turnbull AP, Ball LJ, Schoch GA, et al. Structural diversity in the RGS domain and its interaction with heterotrimeric G protein alpha-subunits. Proc Natl Acad Sci USA 2008; 105:6457 - 6462
- Liu W, Northup JK. The helical domain of a G protein alpha subunit is a regulator of its effector. Proc Natl Acad Sci USA 1998; 95:12878 - 12883
- Day PW, Tesmer JJ, Sterne-Marr R, Freeman LC, Benovic JL, Wedegaertner PB. Characterization of the GRK2 binding site of Galphaq. J Biol Chem 2004; 279:53643 - 53652