Abstract
Drebrin A is one of the most abundant neuron-specific binding proteins of F-actin and its expression is increased in parallel with synapse formation. Drebrin A is particularly concentrated in dendritic spines, postsynaptic sides of excitatory glutamatergic synapses. More recently, Ferhat and colleagues reported the functional role of drebrin A in regulating synaptic transmission. Indeed, our study showed that overexpression of drebrin A induced an increase of glutamatergic but not GABAergic synapses and resulted in the alteration of the normal excitatory-inhibitory ratio in favour of excitation in mature hippocampal neurons. Downregulation of drebrin A expression by antisense oligonucleotides resulted in the decrease of both miniature- glutamatergic and GABAergic synaptic activities without affecting the excitatory-inhibitory ratio. Studies performed in heterologous cells revealed that drebrin A reorganized the actin filaments and stabilized them and that these effects are depend upon its actin-binding domain. These results suggest that drebrin A regulates dendritic spine morphology, size and density, presumably via regulation of actin cytoskeleton remodeling and dynamics. These data demonstrate for the first time that an actin-binding protein such as drebrin A regulates both glutamatergic and GABAergic synaptic transmissions, probably through an increase of active synaptic site density for glutamatergic transmission and through homeostatic mechanisms for the GABAergic one.
The majority of excitatory glutamatergic synapses in the CNS are found on dendritic spines.Citation1 These small protrusions emerging from dendritic shafts constitute sites for the development of neuronal networks and it is believed to provide a cellular substrate for synaptic plasticity.Citation2 Several studies have shown that spines are very dynamic structures, and their shape, size and number change during development and adulthood. During development, dendritic protrusions initiate out as filopodia, which mature into spines.Citation3 In adults, these changes are modulated by synaptic activity and plasticity,Citation4–Citation6 and also associated with learning,Citation7 aging,Citation8 as well as diseases such as mental retardation.Citation9
Actin filaments are the major cytoskeletal components of dendritic spines.Citation10,Citation11 These actin filaments appeared to be the key target of molecular mechanisms modulating spine plasticity because drugs that inhibit actin dynamics caused a loss of spines and inhibition of their motility.Citation12 Time-lapse studies of neurons expressing actin tagged with green fluorescent protein (GFP-actin) revealed that actin-based plasticity in dendritic spines is activity dependent.Citation12 Consistent with this observation, it has been shown that long-term potentiation (LTP), a known form of experimental synaptic plasticity, is accompanied by enhanced F-actin content in dendritic spines in vivoCitation13 and in vitro.Citation14 Thus, the identification of the molecular basis involved in the spine plasticity are essential to understand the mechanisms of synaptic plasticity as well as some neurological disorders such as mental retardation.
The properties of actin cytoskeleton are determined by several proteins that bind to actin filaments. The adult isoform of drebrin, drebrin A (DA), a major neuron specific binding protein of F-actin, emerges as a candidate protein that confers particular characteristics for actin cytoskeleton of dendritic spines.Citation15 This is because DA is specifically localized at dendritic spines of mature neurons,Citation16,Citation17 and is shown to inhibit the actin-binding activity of tropomyosin, fascin and α-actinin.Citation18,Citation19 In vitro, DA also inhibits the interaction between actin and myosin,Citation16,Citation20 suggesting that it possibly regulates actin filament contractility. In fibroblast cells, the overexpression of DA induces reorganization of actin filaments, leading to alteration in their cell shape.Citation21 In mature cortical neurons, the overexpression of DA causes elongation of their dendritic spines.Citation21 Furthermore, the reduction of DA expression by antisense oligonucleotide treatment in developing hippocampal neurons significantly decreases the width and density of filopodia-spines.Citation22,Citation23
Beside its role in cell shape and dendritic spine plasticity, DA may play a role in synaptic function. Indeed, it has been shown that DA induces spinous clustering of the post-synaptic density (PSD) scaffold protein, PSD-95,Citation22 as well as activity-dependent synaptic targeting of NMDA receptors.Citation23 Upon induction of LTP in the hippocampus, drebrin expression is enhanced within dendritic spines.Citation13 Furthermore, the reduction of DA mediated by antisense oligonucleotides causes cognitive deficits.Citation24 These observations also correlate well with a major loss of DA in dendritic spines of patients with Alzheimer's disease and Down's syndrome.Citation25–Citation27 Studies using animal model of Alzheimer's disease revealed also an alteration in DA levels.Citation28 The level of reduction of DA within dendritic spines seems to correlate well with the severity of cognitive impairment.Citation28 Significant decreases in DA levels were also reported in normal aging.Citation26 Altogether, these observations support strongly that the content of DA in dendritic spines is strongly associated with synaptic function.
To evaluate this hypothesis, Ivanov et al.Citation29 studied the effects of DA on the regulation of dendritic spine plasticity and analyzed its electrophysiological consequences of this regulation in mature cultured hippocampal neurons. The results showed that postsynaptic expression of GFP-tagged DA increases dendritic spine length, size and density and that these effects require the actin-binding domain of DA. Studies in heterologous cells revealed that DA reorganized the actin filaments and stabilized them and that these effects were also mediated by its actin-binding domain. Taken together, these findings show that DA regulates dendritic spine plasticity, presumably via regulation of actin cytoskeleton reorganization and dynamics. The close apposition of the presynaptic marker such as synaptophysin or vGlut1 to the long spines-induced by DA suggested the existence of functional excitatory synaptic contacts. Furthermore, we showed that DA increases the density of glutamatergic synapses. Electrophysiological data showed that overexpression of DA increased the glutamatergic synaptic transmission, probably through an increase of active synaptic site density. Surprisingly, enhanced expression of DA also affected the frequency, amplitude and kinetics of miniature inhibitory postsynaptic currents, despite the fact that GABAergic synapse density and transmission efficacy were not modified. Thus, DA increases the glutamatergic to GABAergic synapse ratio and leads to alteration of the normal excitatory-inhibitory balance in favor of excitation. These observations are fundamental because the excitatory to inhibitory synapse ratio is believed to be critical for normal computation of neuronal excitation and is generally kept constant by a homeostatic feedback mechanism.Citation30–Citation34
The identification and characterization of some factors that control the overall change in the ratio of excitatory/inhibitory synapses number and activity have only recently been discovered. Indeed, several studies have involved the synaptic cell adhesion molecules called neuroligin proteins (NLG) and the scaffolding postsynaptic density protein, PSD-95.Citation35,Citation36 The importance of our findings is emphasized by the recent discovery that drebrin level is increased in the superior frontal cortex in neurological disorders accompanied by mild cognitive impairment (MCI), where the synaptic function is known to be reduced.Citation28 Thus, these newly discovered mechanisms could provide an important implication in neurological disorders. An alteration in the ratio excitation/inhibition synaptic activities was also suggested in several neurodevelopmental psychiatric disorders, including autism and some forms of mental retardation.Citation35–Citation37 This notion is emphasized by the numerous studies showing that chromosomal reorganizations in areas that harbor the NLG1, NLG2 and PSD-95 genesCitation38–Citation40 and mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4,Citation41–Citation44 have been associated in autism. Furthermore, an alteration in PSD-95 expression was also been implicated in fragile-X syndrome.Citation45 It has been shown that the overexpression of DA induces the accumulation of PSD-95 into dendritic filopodia.Citation46 In addition, the synaptic clustering of DA is necessary for that of PSD95 in developing neurons.Citation22 Taken together, it is tempting to speculate that DA might be also altered in fragile-X-syndrome leading to an alteration of PSD-95 expression. So, further analysis of the cellular, molecular and physiological defects caused by DA expression level in vitro may, therefore, provide a useful framework for understanding the physiopathological defects of fragile-X syndrome as well as other neurological disorders.
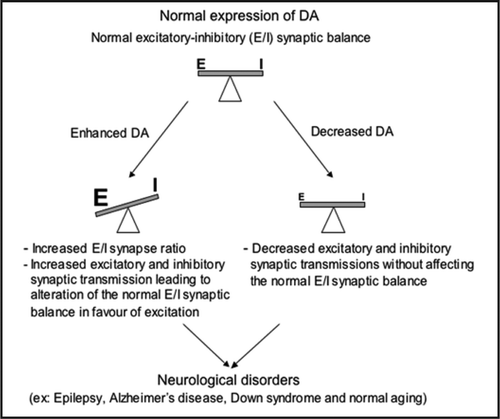
In our study, we investigated also the functional consequences of reduced DA expression in mature cultured hippocampal neurons. Indeed, electrophysiological data showed that the downregulation of DA results into the reduction of both glutamatergic and GABAergic synaptic transmissions. Despite these synaptic transmission changes, the excitatory-inhibitory ratio was not altered by the 73% reduction of DA expression. These data indicated that 27% of residual DA was sufficient to maintain the functional balance between excitation and inhibition. Thus, the remaining DA expression can participate to the homeostatic mechanism that maintains the normal excitatory-inhibitory functional balance. The importance of our results is emphasized also by the recent discovery that a decrease level in DA content is reported in the superior temporal cortex from no cognitive impairment to MCI and to Alzheimer's disease. Furthermore, the level of DA expression has been reported to correlate well with the severity of cognitive impairment.Citation28 These observations suggested that a critical amount of DA protein might be required for normal function. Therefore, the misregulation of the actin-based machinery by the loss of DA protein will lead to alteration of synaptic transmission underlying the cognitive impairment accompanying neurological disorders.Citation37 Thus, we propose a model (see ) in which alterations in the amounts of DA (overexpression or underexpresssion) might result in strengthening or weakening of synaptic transmission and in turn could modulate the normal excitatory-inhibitory balance. In all cases, these synaptic alterations result in synaptic dysfunction, which according to different brain regions involved, may underlie some neurological disorders.
In summary, the work of Ivanov and colleaguesCitation29 provides the first evidence for the involvement of DA in the regulation of excitatory and inhibitory synaptic transmission in mature hippocampal neurons. Future work on the identification and characterization of new synaptic partners of DA involved in synaptogenesis is needed to clarify the physiological mechanisms of synaptic plasticity as well as the cellular, molecular and physiological defects found in neurological disorders.
Figures and Tables
Figure 1 Relative levels of DA expression control synaptic activity leading or not to the alteration of the normal excitatory-inhibitory (E/I) synaptic activity ratio. DA alterations (overexpression or underexpresssion) might result in either strengthening or weakening of synaptic transmission, which in turn could regulate the normal excitatory-inhibitory balance. In all cases, these synaptic alterations lead in synaptic dysfunction, which according to different brain regions involved, could underlies complex psychiatric disorders such as autism and mental retardation or the cognitive impairment accompanying normal aging and neurological disorders, including Epilepsy, Alzheimer's disease and Down's syndrome.
Acknowledgements
This work was supported by grants from the Institut National de la Santé et de la Recherche Médicale (INSERM) and the Centre National de la Recherche Scientifique (CNRS).
References
- Harris KM, Kater SB. Dendritic spines: cellular specializations imparting both stability and flexibility to synaptic function. Annu Rev Neurosci 1994; 17:341 - 371
- Yuste R, Bonhoeffer T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu Rev Neurosci 2001; 24:1071 - 1089
- Hering H, Sheng M. Dendritic spines: structure, dynamics and regulation. Nat Rev Neurosci 2001; 2:880 - 888
- Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature 1999; 399:66 - 70
- Maletic-Savatic M, Malinow R, Svoboda K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science 1999; 283:1923 - 1927
- Toni N, Buchs PA, Nikonenko I, Bron CR, Muller D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature 1999; 402:421 - 425
- Moser MB, Trommald M, Andersen P. An increase in dendritic spine density on hippocampal CA1 pyramidal cells following spatial learning in adult rats suggests the formation of new synapses. Proc Natl Acad Sci USA 1994; 91:12673 - 12675
- Geinisman Y, de Toledo-Morrell L, Morrell F, Persina IS, Rossi M. Age-related loss of axospinous synapses formed by two afferent systems in the rat dentate gyrus as revealed by the unbiased stereological dissector technique. Hippocampus 1992; 2:437 - 444
- Purpura DP. Dendritic spine “dysgenesis” and mental retardation. Science 1974; 186:1126 - 1128
- Fifkova E, Delay RJ. Cytoplasmic actin in neuronal processes as a possible mediator of synaptic plasticity. J Cell Biol 1982; 95:345 - 350
- Matus A, Ackermann M, Pehling G, Byers HR, Fujiwara K. High actin concentrations in brain dendritic spines and postsynaptic densities. Proc Natl Acad Sci USA 1982; 79:7590 - 7594
- Matus A. Actin-based plasticity in dendritic spines. Science 2000; 290:754 - 758
- Fukazawa Y, Saitoh Y, Ozawa F, Ohta Y, Mizuno K, Inokuchi K. Hippocampal LTP is accompanied by enhanced F-actin content within the dendritic spine that is essential for late LTP maintenance in vivo. Neuron 2003; 38:447 - 460
- Okamoto K, Nagai T, Miyawaki A, Hayashi Y. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nat Neurosci 2004; 7:1104 - 1112
- Sekino Y, Kojima N, Shirao T. Role of actin cytoskeleton in dendritic spine morphogenesis. Neurochem Int 2007; 51:92 - 104
- Hayashi K, Ishikawa R, Ye LH, He XL, Takata K, Kohama K, et al. Modulatory role of drebrin on the cytoskeleton within dendritic spines in the rat cerebral cortex. J Neurosci 1996; 16:7161 - 7170
- Aoki C, Sekino Y, Hanamura K, Fujisawa S, Mahadomrongkul V, Ren Y, et al. Drebrin A is a postsynaptic protein that localizes in vivo to the submembranous surface of dendritic sites forming excitatory synapses. J Comp Neurol 2005; 483:383 - 402
- Ishikawa R, Hayashi K, Shirao T, Xue Y, Takagi T, Sasaki Y, et al. Drebrin, a development-associated brain protein from rat embryo, causes the dissociation of tropomyosin from actin filaments. J Biol Chem 1994; 269:29928 - 29933
- Sasaki Y, Hayashi K, Shirao T, Ishikawa R, Kohama K. Inhibition by drebrin of the actin-bundling activity of brain fascin, a protein localized in filopodia of growth cones. J Neurochem 1996; 66:980 - 988
- Ishikawa R, Katoh K, Takahashi A, Xie C, Oseki K, Watanabe M, et al. Drebrin attenuates the interaction between actin and myosin-V. Biochem Biophys Res Commun 2007; 359:398 - 401
- Hayashi K, Shirao T. Change in the shape of dendritic spines caused by overexpression of drebrin in cultured cortical neurons. J Neurosci 1999; 19:3918 - 3925
- Takahashi H, Sekino Y, Tanaka S, Mizui T, Kishi S, Shirao T. Drebrin-dependent actin clustering in dendritic filopodia governs synaptic targeting of postsynaptic density-95 and dendritic spine morphogenesis. J Neurosci 2003; 23:6586 - 6595
- Takahashi H, Mizui T, Shirao T. Downregulation of drebrin A expression suppresses synaptic targeting of NMDA receptors in developing hippocampal neurones. J Neurochem 2006; 97:110 - 115
- Kobayashi R, Sekino Y, Shirao T, Tanaka S, Ogura T, Inada K, et al. Antisense knockdown of drebrin A, a dendritic spine protein, causes stronger preference, impaired pre-pulse inhibition, and an increased sensitivity to psychostimulant. Neurosci Res 2004; 49:205 - 217
- Harigaya Y, Shoji M, Shirao T, Hirai S. Disappearance of actin-binding protein, drebrin, from hippocampal synapses in Alzheimer's disease. J Neurosci Res 1996; 43:87 - 92
- Hatanpää K, Isaacs KR, Shirao T, Brady DR, Rapoport SI. Loss of proteins regulating synaptic plasticity in normal aging of the human brain and in Alzheimer disease. J Neuropathol Exp Neurol 1999; 58:637 - 643
- Shim KS, Lubec G. Drebrin, a dendritic spine protein, is manifold decreased in brains of patients with Alzheimer's disease and Down syndrome. Neurosci Lett 2002; 324:209 - 212
- Kojima N, Shirao T. Synaptic dysfunction and disruption of postsynaptic drebrin-actin complex: a study of neurological disorders accompanied by cognitive deficits. Neurosci Res 2007; 58:1 - 5
- Ivanov A, Esclapez M, Pellegrino C, Shirao T, Ferhat L. Drebrin A regulates dendritic spine plasticity and synaptic function in mature cultured hippocampal neurons. J Cell Sci 2009; 122:524 - 534
- Burrone J, O'Byrne M, Murthy VN. Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature 2002; 420:414 - 418
- Hausser M, Spruston N, Stuart GJ. Diversity and dynamics of dendritic signaling. Science 2000; 290:739 - 744
- Knott GW, Quairiaux C, Genoud C, Welker E. Formation of dendritic spines with GABAergic synapses induced by whisker stimulation in adult mice. Neuron 2002; 34:265 - 273
- Liu G. Local structural balance and functional interaction of excitatory and inhibitory synapses in hippocampal dendrites. Nat Neurosci 2004; 7:373 - 379
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci 2004; 5:97 - 107
- Levinson JN, El-Husseini A. Building excitatory and inhibitory synapses: balancing neuroligin partnerships. Neuron 2005a; 48:171 - 174
- Levinson JN, El-Husseini A. New players tip the scales in the balance between excitatory and inhibitory synapses. Mol Pain 2005b; 1:12
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav 2003; 2:255 - 267
- Konstantareas MM, Homatidis S. Chromosomal abnormalities in a series of children with autistic disorder. J Autism Dev Disord 1999; 29:275 - 285
- Auranen M, Vanhala R, Varilo T, Ayers K, Kempas E, Ylisaukko-Oja T, et al. A genomewide screen for autism-spectrum disorders: evidence for a major susceptibility locus on chromosome 3q25-7. Am J Hum Genet 2002; 71:777 - 790
- Zoghbi HY. Postnatal neurodevelopmental disorders: meeting at the synapse?. Science 2003; 302:826 - 830
- Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC, et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet 2003; 34:27 - 29
- Chih B, Afridi SK, Clark L, Scheiffele P. Disorder-associated mutations lead to functional inactivation of neuroligins. Hum Mol Genet 2004; 13:1471 - 1477
- Comoletti D, De Jaco A, Jennings LL, Flynn RE, Gaietta G, Tsigelny I, et al. The Arg451Cys-neuroligin-3 mutation associated with autism reveals a defect in protein processing. J Neurosci 2004; 24:4889 - 4893
- Laumonnier F, Bonnet-Brilhault F, Gomot M, Blanc R, David A, Moizard MP, et al. X-linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family. Am J Hum Genet 2004; 74:552 - 557
- Todd PK, Mack KJ, Malter JS. The fragile X mental retardation protein is required for type-I metabotropic glutamate receptor-dependent translation of PSD-95. Proc Natl Acad Sci USA 2003; 100:14374 - 14378
- Mizui T, Takahashi H, Sekino Y, Shirao T. Overexpression of drebrin A in immature neurons induces the accumulation of F-actin and PSD-95 into dendritic filopodia, and the formation of large abnormal protrusions. Mol Cell Neurosci 2005; 30:630 - 638