Abstract
Many functions have been ascribed to polyamines, but there has been no clear identification of a unique role for spermine. The Gy mouse has a deletion of part of the X chromosome that includes the SMS gene encoding spermine synthase. Tissues from male Gy mice have no spermine but increased spermidine. They have multiple abnormalities including a tendency to sudden death, small size, circling behavior and other neurological symptoms, sterility and deafness. These changes are reversed by breeding with mice expressing a spermine synthase transgene. Detailed studies of hearing in Gy mice show that the absence of spermine synthase leads to loss of the endocochlear potential. Since this potential requires the cochlear lateral wall-specific Kir4.1 channel, regulation by spermine of transport via these channels appears to be an essential function. A similar spermine-related defect in the functioning of cardiac Kir channels could account for arrhythmias leading to sudden death. The effect of the absence of spermine on glutamate receptor ion channels in the brain may account for the neurological symptoms and could contribute to the lack of fertility and normal growth but more direct effects on gene expression are also possible. Advantages and limitations of the Gy model are discussed.
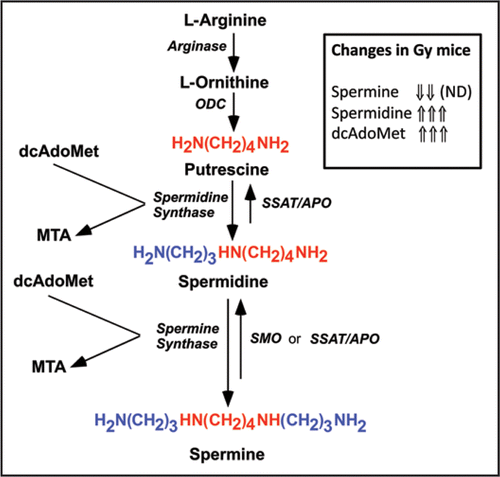
The structures of the polyamines (putrescine, spermidine and spermine) and the enzymes responsible for their synthesis and interconversion are shown in . Polyamines are clearly essential components of mammalian cells since inactivation of genes encoding early steps in the pathway for their biosynthesis is lethal. However, these studies do not provide a clear explanation of the need for spermine. Polyamine function has been the topic of a myriad of publications and numerous roles have been described. Briefly, these can be summarized as affecting the gene expression, either directly or by altering the expression of proteins that act as transcription and translation factors, modifying signaling pathways including kinases and phosphatases and acting as regulators of ion channels (reviewed in refs. Citation1–Citation5). A multitude of secondary effects arise from these sources. A unique function of spermidine in eukaryotes is that it is used as the precursor of hypusine, a post-translational modification of eIF-5A. This modification is essential for eIF-5A function(s). Although these functions are not well understood, the modification is essential for viability and at least partially accounts for the absolute requirement for spermidine.Citation6,Citation7 However, spermine can only be used for hypusine synthesis after back-conversion into spermidine. All of the other polyamine functions that have been described can be fulfilled by either spermidine or spermine albeit with different affinity and potency.
Furthermore, many microorganisms do not contain spermine and yeast is viable and grows normally even when the spermine synthase gene (SMS) is deleted.Citation8 In plants, SMS is also dispensable although there is another gene encoding the synthesis of the isomer, thermospermine, and this product may substitute for spermine.Citation9 It seems unlikely that genes leading to the production of spermine would have been retained without their imparting some benefit and it is possible that a requirement may be seen in yeast and plants if placed under a stress but this have not yet been demonstrated.
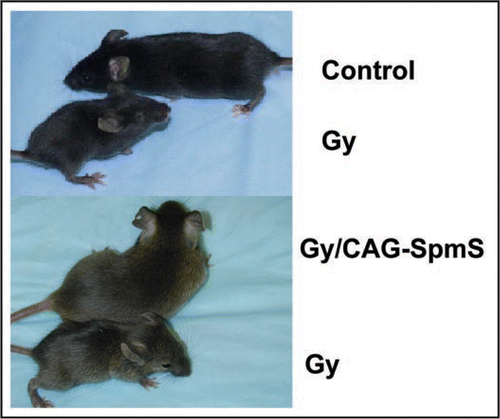
In mammals however, there is more compelling evidence that spermine plays a unique function. A strain of mouse called Gy has a deletion of the X chromosome that eliminates the SMS gene.Citation10,Citation11 Male mice with this deletion have a severe phenotype as described below (). A rare X-linked recessive human disease termed Snyder-Robinson syndrome (SRS) is caused by SMS gene mutations that greatly reduce, but do not eliminate all spermine synthase activity.Citation12–Citation14 Affected males have mental retardation, hypotonia and movement disorders as well as bone-related abnormalities including a marfanoid habitus, skeletal defects, osteoporosis and facial asymmetry. Also, although the mechanism is unclear, it has been reported that patients with Alzheimer's disease have reduced spermine and increased spermidine levels in the brain.Citation15
Tissues in Gy mice have no spermine and cultured cells from SRS patients have a reduction in spermine. This is certainly consistent with the concept of a unique function for spermine. This is the current working hypothesis but it should be pointed out there are two other less likely explanations that have not been completely ruled out. Firstly, there is substantial increase in spermidine and in total polyamine content. (It appears that the reduction in spermine leads to a stimulation of the earlier steps in the pathway, and thus the rise is spermidine is greater than the decrease in spermine). Secondly, there is a huge rise in the content of the aminopropyl-donor dcAdoMet (see ) due to an increase in its synthesis and lack of conversion into spermine. It is possible that the rise in dcAdoMet or spermidine affects the phenotype.
The Gy mouse model provides an opportunity to probe unique functions of spermine but it is not an ideal model. The phenotype of these mice includes reduced bone density, a tendency to sudden death, small size, a tendency to circling behavior (hence the name Gyro) and other neurological symptoms, sterility and deafness (). We have shown that all of these attributes, except for the effect on bone, are reversed by breeding with mice expressing a spermine synthase transgene (CAG-SMS).Citation16,Citation17 The primary cause of the poor bone development in Gy mice is that the X chromosomal deletion also inactivates a gene called Phex, which regulates phosphate metabolism. Other deletions of Phex that do not extend into the SMS or inactivating point mutations of Phex also cause similar changes in bone but none of the changes in other organs described above that are reversed by provision of spermine synthase. Thus, it cannot be concluded from this model that spermine is required for normal bone development and maturation but this cannot really be ruled out and requires further work since the human SRS phenotype suggests a skeletal involvement.
Our recently published studies demonstrate clearly that normal synthesis of spermine is needed for hearing.Citation17 The Gy mice are totally deaf; distortion product otoacoustic emission testing showed no difference between the response of Gy mice and the noise floor. There was an almost complete loss of the endocochlear potential. All of these changes were reversed by breeding with the CAG-SMS mice, which express spermine synthase under the control of a composite CMV-IE enhancer/chicken beta-actin promoter that gives ubiquitous but unregulated expression of spermine synthase.Citation18 Although it was not possible to measure the spermine levels in the inner ear due to the small amount of tissue, it is likely, based on our measurements of many other organs,Citation18,Citation19 that spermine was absent in this organ in the Gy mice and restored to normal levels by the expression of the transgene. A plausible explanation for these results is that the cochlear lateral wall-specific Kir4.1 channel, which is know to be one of the channels subject to strong inward rectification by polyamines with spermine being the most potent,Citation4,Citation20,Citation21 is critical to maintain the endocochlear potential and that spermine is essential for this purpose. These results provide strong support for the physiological importance of regulation of inward rectification of Kir channels by spermine and establish a clear function for normal polyamine levels in hearing.Citation17
Another phenotype of the Gy mice is a tendency to sudden death. The reason for this is not fully understood at present but a reasonable hypothesis is that cardiac arrhythmias lead to sudden death. We have preliminary evidence that the Gy mice show an altered electrocardiogram indicating abnormal cardiac electrical activity. This could also be due to an altered activity of the inwardly rectifying Kir channels located in the myocytes.Citation22,Citation23 Further study is needed to establish the importance of normal spermine levels in ion movement across the membrane in these cells but this is an important area for further research.
Interactions with other types of ion channels may underlie some of the other defects in Gy mice since spermine is the most potent polyamine in these reactions. Polyamines have been shown to affect glutamate receptor ion channels (NMDA, AMPA and kainate receptors), certain connexin-linked gap junctions and some other channels that are involved in intracellular calcium signaling or Na+ transport.Citation5,Citation24–Citation27 The neurological deficiencies in Gy mice (and in SRS patients) could also be related to a lack of effects of spermine on glutamate receptors that mediate synaptic plasticity and excitatory synaptic transmission in the mammalian brain. However, it is certainly possible that some effects are related to altered gene expression due to the changed polyamine content altering metabolites in the brain and elsewhere. In preliminary experiments using magnetic resonance spectroscopy, we have observed a decrease in the taurine:creatine + phosphocreatine ratio in the hippocampus region of the brains of Gy mice that is reversed in the Gy/CAG-SMS crosses.
The sterility of the Gy mice is not due solely to their small size. There is a striking alteration in the testicular morphology with most of the cells in the seminiferous tubules remaining as spermatogonia or early stage primary spermatocytes with an almost total absence of mature spermatozoa.Citation16 There is independent evidence that strict control of polyamine levels is needed for testicular function. Overexpression of ODC is associated with a loss of fertilityCitation28 and there are haploid germ cell specific forms of both antizyme and antizyme inhibitor, which provide tight regulation of ODC.Citation29 However, it is not certain that the defect in sperm development in the Gy mice is solely due to local lack of correct polyamine levels. There appears to be a reduction in the number of Leydig cells in the testes and brain-related endocrine defects may also contribute to developmental defects.Citation16 Cell cultures from Gy and rescued CAG-SMS/Gy mice are a promising tool to attack this problem.
Direct studies of ion channel activity and proteomic analysis of altered protein content in the Gy mice are promising avenues for the further identification of spermine functions. However, this model has many disadvantages in addition to the deletion of the Phex gene described above and the tendency to sudden death. A serious problem with the Gy mouse model is that the mice are only viable on the mixed B6C3H background and attempts to transfer the genotype by back-crossing or to generate clean knock outs by inactivating only the spermine synthase have not led to any viable offspring. Such mixed backgrounds pose significant problems for experimentation and greatly increase individual variability. The studies already carried out show that spermine deficiency affects a number of organs including reproductive tissue, the inner ear, the brain and heart. It should be possible to generate more useful experimental models to address the critical function of spermine by selectively inactivating the spermine synthase gene in particular cell types. Although there is some concern that these models may be compromised by uptake of spermine from other cells, the studies with Gy mice suggest that this is not likely to occur to any major extent. All tissues from Gy mice that have been examined so far have undetectable levels of spermine despite a diet that contains this polyamine.Citation16,Citation19 Even after increasing the spermine content in the diet or administration of spermine by injection of the maximally tolerated dose there was only a very limited uptake.Citation17
The CAG-SMS mice were originally derived on the B6D2 background but have been backcrossed to inbred B6 strain.Citation18 Since the crosses of these mice with Gy mice are viable and fertile, it should be possible by continued backcrossing to B6 to identify the gene(s) that are present in the B6C3H strain that allow the male Gy spermine synthase mice to survive even in the absence of spermine.
Finally, the results showing a lack of spermine in the tissues of Gy mice, even after feeding them with diets that contain substantial amounts of spermine or injection of spermine,Citation17 raise major questions concerning the polyamine transport system. Such systems have been identified for the movement of polyamines across the intestinal wall and uptake into many different cell types.Citation30,Citation31 The reason that this system does not contribute significantly to spermine content is unclear but may be related to the elevated spermidine content. Overcoming the uptake block would enable experimentation to reverse the Gy mouse phenotype to be carried out by providing spermine rather than a spermine synthase transgene and, more importantly, might suggest a therapeutic approach to treatment of SRS patients, particularly if uptake into the brain can be achieved.
Figures and Tables
Figure 1 Polyamine structure and biosynthesis/interconversion. The abbreviations used are dcAdoMet, decarboxylated S-adenosylmethionine; MTA, 5′-methylthioadenosine; ODC, L-ornithine decarboxylase; APO, acetylpolyamine oxidase; SMO spermine oxidase; SSAT, spermidine/spermine-N1-acetyltransferase; ND, not detected.
Acknowledgements
Research on polyamines in the authors' laboratory is supported by grants CA-018138 and GM-26290 from the National Institutes of Health, Bethesda, MD.
References
- Gerner EW, Meyskens FL Jr. Polyamines and cancer: old molecules, new understanding. Nat Rev Cancer 2004; 4:781 - 792
- Terui Y, Higashi K, Taniguchi S, Shigemasa A, Nishimura K, Yamamoto K, et al. Enhancement of the synthesis of RpoN, Cra and H-NS by polyamines at the level of translation in Escherichia coli cultured with glucose and glutamate. J Bacteriol 2007; 189:2359 - 2368
- Ray RM, Bhattacharya S, Johnson LR. EGFR plays a pivotal role in the regulation of polyamine-dependent apoptosis in intestinal epithelial cells. Cell Signal 2007; 19:2519 - 2527
- Kurata HT, Diraviyam K, Marton LJ, Nichols CG. Blocker protection by short spermine analogs: refined mapping of the spermine binding site in a Kir channel. Biophys J 2008; 95:3827 - 3839
- Jin L, Miyazaki M, Mizuno S, Takigawa M, Hirose T, Nishimura K, et al. The pore region of N-methyl-D-aspartate receptors differentially influences stimulation and block by spermine. J Pharmacol Exp Ther 2008; 327:68 - 77
- Hyvonen MT, Keinanen TA, Cerrada-Gimenez M, Sinervirta R, Grigorenko N, Khomutov AR, et al. Role of hypusinated eukaryotic translation initiation factor 5A in polyamine depletion-induced cytostasis. J Biol Chem 2007; 282:34700 - 34706
- Chattopadhyay MK, Park MH, Tabor H. Hypusine modification for growth is the major function of spermidine in Saccharomyces cerevisiae polyamine auxotrophs grown in limiting spermidine. Proc Natl Acad Sci USA 2008; 105:6554 - 6559
- Hamasaki-Katagiri N, Katagiri Y, Tabor CW, Tabor H. Spermine is not essential for growth of Saccharomyces cerevisiae: identification of the SPE4 gene (spermine synthase) and characterization of a spe4 deletion mutant. Gene 1998; 210:195 - 210
- Knott JM, Romer P, Sumper M. Putative spermine synthases from Thalassiosira pseudonana and Arabidopsis thaliana synthesize thermospermine rather than spermine. FEBS Lett 2007; 581:3081 - 3086
- Lyon MF, Scriver CR, Baker LR, Tenenhouse HS, Kronick J, Mandla S. The Gy mutation: another cause of X-linked hypophosphatemia in mouse. Proc Natl Acad Sci USA 1986; 4899 - 4903
- Meyer RA Jr, Henley CM, Meyer MH, Morgan PL, McDonald AG, Mills C, et al. Partial deletion of both the spermine synthase gene and the Pex gene in the x-linked hypophosphatemic, Gyro (Gy) mouse. Genomics 1998; 48:289 - 295
- Cason AL, Ikeguchi Y, Skinner C, Wood TC, Lubs HA, Martinez F, et al. X-Linked spermine synthase gene (SMS) defect: The first polyamine deficiency syndrome. Eur J Human Genet 2003; 11:937 - 944
- de Alencastro G, McCloskey DE, Kliemann SE, Maranduba CM, Pegg AE, Wang X, et al. New SMS mutation leads to a striking reduction in spermine synthase protein function and a severe form of Snyder-Robinson X-linked recessive mental retardation syndrome. J Med Genet 2008; 45:539 - 543
- Becerra-Solano LE, Butler J, Castaneda-Cisneros G, McCloskey DE, Wang X, Pegg AE, et al. A missense mutation, p.V132G, in the X-linked spermine synthase gene (SMS) causes Snyder-Robinson syndrome. Am J Med Genet A 2009; 149A:328 - 335
- Morrison LD, Kish SJ. Brain polyamine levels are altered in Alzheimer's disease. Neurosci Lett 1995; 197:5 - 8
- Wang X, Ikeguchi Y, McCloskey DE, Nelson P, Pegg AE. Spermine synthesis is required for normal viability, growth and fertility in the mouse. J Biol Chem 2004; 49:51370 - 51375
- Wang X, Levic S, Gratton MA, Doyle KJ, Yamoah EN, Pegg AE. Spermine synthase deficiency leads to deafness and a profound sensitivity to alpha-difluoromethylornithine. J Biol Chem 2009; 284:930 - 937
- Ikeguchi Y, Wang X, McCloskey DE, Coleman CS, Nelson P, Hu G, et al. Characterization of transgenic mice with widespread overexpression of spermine synthase. Biochem J 2004; 381:701 - 707
- Mackintosh CA, Pegg AE. Effect of spermine synthase deficiency on polyamine biosynthesis and content in mice and embryonic fibroblasts and the sensitivity of fibroblasts to 1,3-bis(2-chloroethyl)-N-nitrosourea. Biochem J 2000; 351:439 - 447
- Phillips LR, Nichols CG. Ligand-induced closure of inward rectifier Kir6.2 channels traps spermine in the pore. J Gen Physiol 2003; 122:795 - 804
- Kurata HT, Marton LJ, Nichols CG. The polyamine binding site in inward rectifier K+ channels. J Gen Physiol 2006; 127:467 - 480
- Lopatin AN, Shantz LM, Mackintosh CA, Nichols CG, Pegg AE. Modulation of potassium channels in the hearts of transgenic and mutant mice with altered polyamine biosynthesis. J Mol Cell Cardiol 2000; 32:2007 - 2024
- Li J, McLerie M, Lopatin AN. Transgenic upregulation of IK1 in the mouse heart leads to multiple abnormalities of cardiac excitability. Am J Physiol 2004; 287:2790 - H802
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev 1999; 51:7 - 61
- Lin X, Veenstra RD. Effect of transjunctional KCl gradients on the spermine inhibition of connexin40 gap junctions. Biophys J 2007; 93:483 - 495
- Fleidervish IA, Libman L, Katz E, Gutnick MJ. Endogenous polyamines regulate cortical neuronal excitability by blocking voltage-gated Na+ channels. Proc Natl Acad Sci USA 2008; 105:18994 - 18999
- Shin J, Shen F, Huguenard J. PKC and polyamine modulation of GluR2-deficient AMPA receptors in immature neocortical pyramidal neurons of the rat. J Physiol 2007; 581:679 - 691
- Halmekytö M, Alhonen L, Wahlfors J, Sinervirta R, Eloranta T, Jänne J. Characterization of a transgenic mouse line overexpressing the human ornithine decarboxylase gene. Biochem J 1991; 278:895 - 898
- Christensen GL, Ivanov IP, Wooding SP, Atkins JF, Mielnik A, Schlegel PN, et al. Identification of polymorphisms and balancing selection in the male infertility candidate gene, ornithine decarboxylase antizyme 3. BMC Med Genet 2006; 7:27
- Larque E, Sabater-Molina M, Zamora S. Biological significance of dietary polyamines. Nutrition 2007; 23:87 - 95
- Seiler N, Delcros JG, Moulinoux JP. Polyamine transport in mammalian cells. An update. Int J Biochem 1996; 28:843 - 861