Abstract
The second messenger cGMP controls cardiovascular and gastrointestinal homeostasis in mammals. However, its physiological relevance in the nervous system is poorly understood.1 Now, we have reported that the cGMP-dependent protein kinase type I (PRKG1) is implicated in the regulation of the timing and quality of sleep and wakefulness.2 Prkg1 mutant mice showed altered distribution of sleep and wakefulness as well as reduction in rapid-eye-movement sleep (REMS) duration and in non-REMS consolidation. Furthermore, the ability to sustain waking episodes was compromised. These observations were also reflected in wheel-running and drinking activity. A decrease in electroencephalogram power in the delta frequency range (1–4 Hz) under baseline conditions was observed, which was normalized after sleep deprivation. Together with the finding that circadian clock amplitude is reduced in Prkg1 mutants these results indicate a decrease of the wake-promoting output of the circadian system affecting sleep. Because quality of sleep might affect learning we tested Prkg1 mutants in several learning tasks and find normal spatial learning but impaired object recognition memory in these animals. Our findings indicate that Prkg1 impinges on circadian rhythms, sleep and distinct aspects of learning.
The mammalian circadian clock influences a multitude of physiological processes such as cardiovascular activity, sleep and wakefulness, metabolism and brain function, thereby optimizing an organism's performance in anticipating changing environmental conditions. To adapt to such changes the phase of the circadian clock can be advanced or delayed e.g., by light acting via the retinohypothalamic tract (RHT) on the suprachiasmatic nuclei (SCN), the main coordinator of circadian rhythms in the brain.Citation3 One of the signaling pathways involved in the light dependent resetting process appears to involve the second messenger cyclic guanosine monophosphate (cGMP). For example, sildenafil (Viagra®), an inhibitor of the cGMP-specific phosphodiesterase PDE5, prevents the hydrolysis of cGMP and as a consequence adaptation to an advancing light schedule (e.g., jet-lag after a flight from the US to Europe) is accelerated.Citation4 In this resetting mechanism the cGMP-dependent protein kinase (PRKG, also known as cGK or PKG) has been implicated as the downstream effector of cGMP.Citation5 From the two PRKG's in mammals, PRKG1 and PRKG2, PRKG2 has been shown to modulate resetting of the circadian clock.Citation6 In contrast, PRKG1 appears to have no major role in this process, because mutant mice lacking Prkg1 in the brain (Prkg1BKO mice) showed normal light-induced phase shifts of the circadian clock as assessed by wheel-running.Citation2 In line with the absence of a behavioral phase shift phenotype, light-induced expression of the light inducible clock genes Per1 and Per2 in the SCN is not altered in mice lacking Prkg1 in the brain as compared to controls ( and B). Interestingly, light induction of cFos is reduced in Prkg1BKO animals after a light pulse at circadian time (CT) 14, but not at CT22 (). In contrast light induced cFos expression is not affected in Prgk2 mutants compared to controls.Citation6 These results indicate distinct roles of PRKG1 and PRKG2 in the SCN and probably also in the rest of the brain. Since FOS is a transcriptional regulator that is involved in cellular mechanisms mediating neuronal excitability and survival,Citation7 it is likely that Prkg1BKO animals display phenotypes related to complex behaviors.
In line with this hypothesis are recent findings from invertebrates indicating that cGMP signaling via PRKGs modulates complex behaviors (reviewed in ref. Citation8). For instance, in insects and nematodes PRKGs are involved in foraging and sensory adaptation.Citation9–Citation12 The Drosophila PRKG has also a function in certain forms of learning and memory,Citation13 and the C. elegans PRKG has been reported to promote a sleep-like state called lethargus.Citation14 In rodents, electrophysiological recordings in brain slices and cultured neurons treated with PRKG activators or inhibitors, and studies with wild-type and Prkg mutant mice, indicated a role of PRKG1 in synaptic plasticity in various brain regions including the hippocampus,Citation15–Citation17 cerebellumCitation18,Citation19 and amygdala.Citation20,Citation21 The analysis of the in vivo significance of mammalian PRKG1 for learning and memory and for other complex behaviors proved to be difficult, because PRKG1 is expressed in many brain regionsCitation22 and conventional Prkg1 null mice die at approximately six weeks of age, presumably due to smooth muscle dysfunction.Citation23,Citation24
To dissect the role of mammalian PRKG1 in complex behaviors, we have generated conditional mouse mutants lacking PRKG1 in the whole nervous system. In one model, PRKG1 was rescued selectively in smooth muscle of Prkg1 null mice (Prkg1SMr miceCitation24). The other model, termed Prkg1 brain knockout (Prkg1BKO), was generated by Cre/lox-assisted neuron-specific inactivation of the Prkg1 gene using the Nes-Cre mouse line.Citation2 Our initial analysis of Prkg1SMr and Prkg1BKO mice revealed that PRKG1 regulates the timing and quality of sleep and wakefulness, while it is dispensable for the basal functions of the sleep homeostat and circadian clock.Citation2 In the absence of PRKG1 in the nervous system, sleep and wakefulness were more fragmented and their distribution over the 24 h of a day was altered. The sleep phenotype correlated well with a redistribution of rest-activity phases as monitored by wheel-running and drinking analysis.Citation2 These findings point to a general behavioral state instability of the Prkg1 mouse mutants and suggest that endogenous PRKG1 is a modifier of behavioral rhythmicity in rodents. Since cGMP-PRKG1 signaling has also been linked to learning and memory, and since the lower sleep quality in Prkg1 mutants might also affect cognition (reviewed in refs. Citation25 and Citation26), we have analyzed learning and memory in these mice.
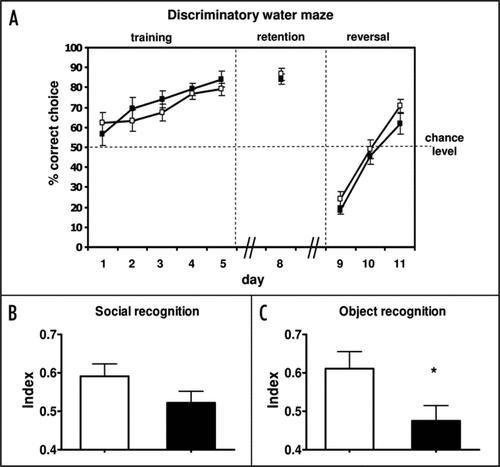
Spatial learning as analyzed in a discriminatory water maze test was not affected in Prkg1BKO mice () and Prkg1SMr mice (data not shown). These results are in line with other reports showing normal spatial learning in mouse mutants of the cGMP-generating enzyme, soluble guanylyl cyclase,Citation27 as well as in hippocampus-specific Prkg1 mutants.Citation17 Social memory was assessed using a social discrimination task. As expected, control mice explored an unfamiliar mouse significantly longer than a familiar one. Interestingly, Prkg1BKO mice discriminated less well between the familiar and the unfamiliar subject than control animals, resulting in a decreased social discrimination index. However, the difference between genotypes did not reach statistical significance (one-tailed t-test, p = 0.07) (). Another assay to test for memory is novel-object recognition, which is based on the innate tendency of rodents to seek novelty.Citation28 In contrast to control mice, Prkg1BKO mice did not distinguish between a familiar and an unfamiliar object after a retention interval of 24 h, resulting in a significantly decreased object recognition index of the mutants compared to controls (one-tailed t-test, p < 0.05) (). Further tests indicated that neither the visual abilities nor the ability to detect social odors were compromised in Prkg1BKO mice (data not shown). We conclude that PRKG1 in the mammalian brain has little relevance for spatial learning, but is involved in the modulation of other cognitive functions such as social and object recognition. Indeed, pharmacological elevation of cGMP levels in rats had no effect on spatial learning, while it improved social and object memory.Citation29,Citation30 In this context it is also of note that a functional circadian system is required for novel-object recognition.Citation31 In addition, a recent analysis of Prkg1SMr mice identified a role of PRKG1 in the formation of fear memory.Citation21 Together, these findings suggest, that circadian rhythms, sleep and aspects of learning intersect in a signal transduction pathway involving PRKG1. It appears that the mammalian cGMP-PRKG1 signaling system contributes to various forms of behavioral plasticity, ranging from the modulation of sleep-wake activity to cognitive performance. Further studies are necessary to dissect a potential cross-talk between cGMP-modulated sleep and cognition.
Figures and Tables
Figure 1 Light induction of Per1, Per2 and cFos in the SCN of Prkg1BKO mice (black bars, genotype: Prkg1L-/L2; Nes-Cretg/0) compared to litter-matched control mice (grey bars, genotype: Prkg1+/L2; Nes-Cretg/0). Animals were kept in a 12 h light-12 h dark cycle with water and food ad libitum. A light pulse of 500 lux intensity and 15-min duration was applied at circadian time (CT) 14 or 22. One hour after the light pulse animals were sacrificed and analyzed for gene expression. Control animals receiving no light pulse were also analyzed for gene expression (white bars). Shown are the expression levels of (A) Per1 mRNA, (B) Per2 mRNA and (C) cFos mRNA in the SCN as detected by RNA in situ hybridization. The data shown represent relative optical densities as determined by the difference of staining in the SCN relative to surrounding tissue not expressing the genes. Values are the means ± SEM of three independent experiments. Significance was determined by student's t-test (*p < 0.05, n = 3). Animals studied were males between 2–4 months of age. All experiments were in accordance with Swiss animal protection law in the declaration of Helsinki.
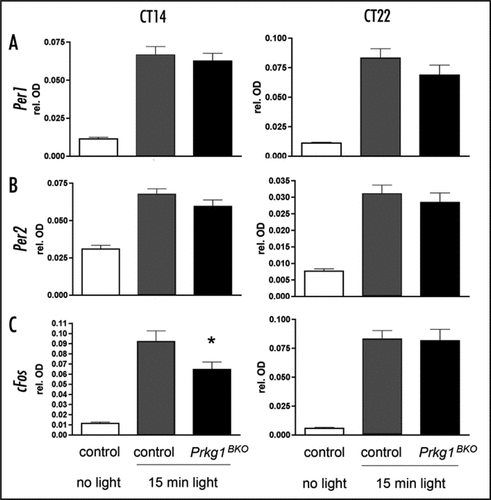
Figure 2 Analysis of cognitive functions of Prkg1BKO mice (black symbols and bars, genotype: Prkg1L-/L2; Nes-Cretg/0) compared to litter-matched control mice (white symbols and bars, genotype: Prkg1+/L2; Nes-Cretg/0). Mice were group-housed with food and water ad libitum, and maintained on a 12 h light-12 h dark cycle. Behavioral tests were performed during the light phase by an observer that was blinded to the genotype of the animals. All experiments were in accordance with German animal protection law. (A) Spatial learning was assessed in a discriminatory water maze task as described.Citation17 Eight- to twelve-month-old female mice (n = 12 per genotype) had to discriminate between two visible platforms: a stable platform remaining in the same position (correct choice) and a platform that was moved in a pseudorandom fashion and submerged when climbed by a mouse. Mice were tested over five training sessions (day 1–5) and again at day 8 (memory retention). To validate spatial learning strategies, the correct platform was then moved to the opposite quadrant (day 9–11, reversal). The number of correct choices is expressed as the percentage of the total number of choices per session (10 trials per session). The escape latencies, i.e., the times required for navigating to one of the two platforms, were not different between genotypes (data not shown). (B) Social memory was tested using the social discrimination paradigmCitation32 in 8- to 9-month-old female mice (n = 22 controls; n = 17 mutants). The procedure consisted of two 4-min exposures of stimulus animals (4-week-old male C56BL/6J mice) to the test animal in a fresh cage to which the test animal had been moved 2 h prior to testing. During the first exposure, one stimulus animal was exposed to the test animal. After a retention interval of 2 h, this stimulus animal was re-exposed to the test animal together with an additional, previously not presented stimulus animal. The duration of investigatory behavior of the test animal towards the stimulus animals was recorded by a trained observer with a hand-held computer. A social recognition index was calculated as time spent investigating the unfamiliar stimulus mouse/time spent investigating both the familiar and unfamiliar stimulus mouse. (C) Object memory was assessed in an object recognition task according to the procedure described by Genoux and co-workersCitation33 using 15-month-old female mice (n = 12 per genotype). Briefly, the mouse was allowed to explore two identical objects three times for 5 min, with an inter-trial interval of 15 min. After a retention interval of 2 h, one of the previously encountered familiar objects was substituted by a new, unfamiliar one, which was again substituted by a novel object after a second retention interval of 24 h. For each retention test the mouse was put again into the test box for 5 min, and exploration time—defined as touching the object with the nose—was recorded by a trained observer with a hand-held computer. An object recognition index was calculated as time spent investigating the unfamiliar object/time spent investigating both the familiar and unfamiliar object. The diagram shows the object recognition index of control and Prkg1BKO mutant mice after a retention interval of 24 h. Note that in this experiment after a retention interval of 2 h the index was not significantly different between genotypes (data not shown). Data obtained in the social and object recognition experiments were analyzed by using the Observer 4.1 Software (Noldus, Wageningen). All data are expressed as mean ± SEM. *p < 0.05 (one-tailed t-test).
Acknowledgements
We like to thank Rüdiger Klein for the Nes-Cre mice, and Anne-Marie Schönegge and Claudia Becker for help with behavioral testing. This research was supported by the DFG and VolkswagenStiftung (Robert Feil), the National Genome Research Network (NGFN), Foerderkennzeichen 01GR0430, and by EUMODIC, LSHG-CT-2006-037188 (Sabine M. Hölter and Wolfgang Wurst), the Dr. Karl Kuhn-Stiftung and fortüne-Programm der Medizinischen Fakultät der Universität Tübingen (grant #1774-0-0) (Susanne Feil), and the Swiss National Science Foundation, the State of Fribourg and EUCLOCK (Urs Albrecht).
Addendum to:
References
- Kleppisch T, Feil R. cGMP signaling in the mammalian brain: role in synaptic plasticity and behaviour. Handb Exp Pharmacol 2009; 191:549 - 579
- Langmesser S, Franken P, Feil S, Emmenegger Y, Albrecht U, Feil R. cGMP-dependent protein kinase type I is implicated in the regulation of the timing and quality of sleep and wakefulness. PLoS ONE 2009; 4:4238
- Hirota T, Fukada Y. Resetting mechanism of central and peripheral circadian clocks in mammals. Zoolog Sci 2004; 21:359 - 368
- Agostino PV, Plano SA, Golombek DA. Sildenafil accelerates reentrainment of circadian rhythms after advancing light schedules. Proc Natl Acad Sci USA 2007; 104:9834 - 9839
- Gillette MU, Mitchell JW. Signaling in the suprachiasmatic nucleus: selectively responsive and integrative. Cell Tissue Res 2002; 309:99 - 107
- Oster H, Werner C, Magnone MC, Mayser H, Feil R, Seeliger MW, et al. cGMP-dependent protein kinase II modulates mPer1 and mPer2 gene induction and influences phase shifts of the circadian clock. Curr Biol 2003; 13:725 - 733
- Zhang J, Zhang D, McQuade JS, Behbehani M, Tsien JZ, Xu M. c-fos regulates neuronal excitability and survival. Nat Genet 2002; 30:416 - 420
- Reaume CJ, Sokolowski MB. cGMP-dependent protein kinase as a modifier of behaviour. Handb Exp Pharmacol 2009; 423 - 443
- Osborne KA, Robichon A, Burgess E, Butland S, Shaw RA, Coulthard A, et al. Natural behavior polymorphism due to a cGMP-dependent protein kinase of Drosophila. Science 1997; 277:834 - 836
- Ben-Shahar Y, Robichon A, Sokolowski MB, Robinson GE. Influence of gene action across different time scales on behavior. Science 2002; 296:741 - 744
- Fujiwara M, Sengupta P, McIntire SL. Regulation of body size and behavioral state of C. elegans by sensory perception and the EGL-4 cGMP-dependent protein kinase. Neuron 2002; 36:1091 - 1102
- L'Etoile ND, Coburn CM, Eastham J, Kistler A, Gallegos G, Bargmann CI. The cyclic GMP-dependent protein kinase EGL-4 regulates olfactory adaptation in C. elegans. Neuron 2002; 36:1079 - 1089
- Mery F, Belay AT, So AK, Sokolowski MB, Kawecki TJ. Natural polymorphism affecting learning and memory in Drosophila. Proc Natl Acad Sci USA 2007; 104:13051 - 13055
- Raizen DM, Zimmerman JE, Maycock MH, Ta UD, You YJ, Sundaram MV, et al. Lethargus is a Caenorhabditis elegans sleep-like state. Nature 2008; 451:569 - 572
- Zhuo M, Hu Y, Schultz C, Kandel ER, Hawkins RD. Role of guanylyl cyclase and cGMP-dependent protein kinase in long-term potentiation. Nature 1994; 368:635 - 639
- Arancio O, Kandel ER, Hawkins RD. Activity-dependent long-term enhancement of transmitter release by presynaptic 3′,5′-cyclic GMP in cultured hippocampal neurons. Nature 1995; 376:74 - 80
- Kleppisch T, Wolfsgruber W, Feil S, Allmann R, Wotjak CT, Goebbels S, et al. Hippocampal cGMP-dependent protein kinase I supports an age- and protein synthesis-dependent component of long-term potentiation but is not essential for spatial reference and contextual memory. J Neurosci 2003; 23:6005 - 6012
- Hartell NA. cGMP acts within cerebellar Purkinje cells to produce long term depression via mechanisms involving PKC and PKG. Neuroreport 1994; 5:833 - 836
- Feil R, Hartmann J, Luo C, Wolfsgruber W, Schilling K, Feil S, et al. Impairment of LTD and cerebellar learning by Purkinje cell-specific ablation of cGMP-dependent protein kinase I. J Cell Biol 2003; 163:295 - 302
- Ota KT, Pierre VJ, Ploski JE, Queen K, Schafe GE. The NO-cGMP-PKG signaling pathway regulates synaptic plasticity and fear memory consolidation in the lateral amygdala via activation of ERK/MAP kinase. Learn Mem 2008; 15:792 - 805
- Paul C, Schoberl F, Weinmeister P, Micale V, Wotjak CT, Hofmann F, et al. Signaling through cGMP-dependent protein kinase I in the amygdala is critical for auditory-cued fear memory and long-term potentiation. J Neurosci 2008; 28:14202 - 14212
- Feil S, Zimmermann P, Knorn A, Brummer S, Schlossmann J, Hofmann F, Feil R. Distribution of cGMP-dependent protein kinase type I and its isoforms in the mouse brain and retina. Neuroscience 2005; 135:863 - 868
- Pfeifer A, Klatt P, Massberg S, Ny L, Sausbier M, Hirneiss C, et al. Defective smooth muscle regulation in cGMP kinase I-deficient mice. EMBO J 1998; 17:3045 - 3051
- Weber S, Bernhard D, Lukowski R, Weinmeister P, Worner R, Wegener JW, et al. Rescue of cGMP kinase I knockout mice by smooth muscle specific expression of either isozyme. Circ Res 2007; 101:1096 - 1103
- Hobson JA, Pace-Schott EF. The cognitive neuroscience of sleep: neuronal systems, consciousness and learning. Nat Rev Neurosci 2002; 3:679 - 693
- Walker MP, Stickgold R. Sleep, memory and plasticity. Annu Rev Psychol 2006; 57:139 - 166
- Koesling D, Mergia E, Taqatqeh F, Mittmann T, Becker A, Hoellt V, et al. Functional roles of isoforms of NO-sensitive guanylyl cyclase. BMC Pharmacol 2007; 7:44
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci 2004; 27:279 - 306
- Prickaerts J, van Staveren WC, Sik A, Markerink-van Ittersum M, Niewohner U, van der Staay FJ, et al. Effects of two selective phosphodiesterase type 5 inhibitors, sildenafil and vardenafil, on object recognition memory and hippocampal cyclic GMP levels in the rat. Neuroscience 2002; 113:351 - 361
- Prickaerts J, Sik A, van Staveren WC, Koopmans G, Steinbusch HW, van der Staay FJ, et al. Phosphodiesterase type 5 inhibition improves early memory consolidation of object information. Neurochem Int 2004; 45:915 - 928
- Ruby NF, Hwang CE, Wessells C, Fernandez F, Zhang P, Sapolsky R, et al. Hippocampal-dependent learning requires a functional circadian system. Proc Natl Acad Sci USA 2008; 105:15593 - 15598
- Engelmann M, Wotjak CT, Landgraf R. Social discrimination procedure: an alternative method to investigate juvenile recognition abilities in rats. Physiol Behav 1995; 58:315 - 321
- Genoux D, Haditsch U, Knobloch M, Michalon A, Storm D, Mansuy IM. Protein phosphatase 1 is a molecular constraint on learning and memory. Nature 2002; 418:970 - 975