Abstract
Strong “alarm odors” emanating from lethally injured conspecifics may indicate an imminent risk of predation to spiny lobsters. In laboratory trials,1 strong conspecific alarm odors elicited avoidance in Panulirus argus, a highly gregarious species that displays collective defense behavior, but not in Panulirus guttatus, a species that tends to aggregate when reproductive activity is high (spring) but not when it is low (late summer) and does not display collective defensive behavior. To reduce predation risk, however, lobsters may automize limbs, thus sustaining non-lethal injuries. I tested the response of these lobsters to scents emanating from intact, lethally-injured, and non-lethally injured conspecifics. In P. argus, these scents elicited, respectively, attraction, avoidance, and a random response, suggesting that, in P. argus, avoidance of conspecific alarm odors depends on their strength. In contrast, P. guttatus lobsters responded at random to scents of lethally injured conspecifics and showed a similar response to scents of intact and non-lethally injured conspecifics in the spring (attraction) and in the summer (random), reflecting the more cryptic defensive behavior of this species. Therefore, both species use conspecific alarm odors for risk-assessment, but each responds to these cues in the most effective way to reduce its risk of predation.
Marine animals that forage at night strongly rely on their chemical senses for assessing predation risk.Citation2 In particular, chemical cues emanating from injured conspecifics (“alarm odors”) can indicate a more imminent risk of predation, especially to animals that live in groups.Citation3,Citation4 Spiny lobsters (Crustacea: Palinuridae) tend to aggregate in diurnal shelters, a social behavior that is mediated by conspecific chemical attraction.Citation5–Citation8 However, different species vary in their degree of gregariousness.Citation9 For example, Caribbean spiny lobsters (Panulirus argus) have a strong tendency to aggregate and use collective defensive behavior to reduce predation risk.Citation10–Citation12 These lobsters, which are highly mobile and forage away from their shelters,Citation10,Citation13 use conspecific chemical cues to find shelter fasterCitation14 and to assess the quality (potential for gregariousness) of shelters.Citation15 Given the strong adaptive value of gregariousness for P. argus,Citation16 these lobsters tend to aggregate irrespective of season or size.Citation5,Citation11,Citation17 In contrast, spotted spiny lobsters (Panulirus guttatus) are sedentary, obligate reef-dwellers that forage close to their shelters.Citation17–Citation19 We recently found that these lobsters tend to aggregate when reproductive activity in the population is high (spring), but not when reproductive activity is low (late summer).Citation11,Citation20 Also importantly, P. guttatus lobsters do not display collective defense behavior and, to reduce predation risk, they hide as deeply as possible in any available crevice and remain still.Citation11,Citation12,Citation18
In a recent study,Citation1 we tested the effect of conspecific alarm odors on the shelter choice by individuals of P. argus and P. guttatus. Each test lobster was free to choose between two identical shelters, one in each arm of a Y-maze. One shelter received plain seawater flowing through a separate head tank while the other shelter received seawater flowing through another head tank that held a lethally injured lobster (one half of a freshly killed conspecific), not visible to the test lobster. P. argus lobsters significantly avoided shelters emanating conspecific alarm odors, whereas the shelter choice by P. guttatus lobsters did not differ significantly from random, suggesting that, in spiny lobsters, decision making upon predation risk-assessment via conspecific alarm odors is related to gregarious behavior.
Although injured lobsters release all kinds of bodily fluids, it was recently shown that, in P. argus, chemicals that act as alarm odors are blood-borne and that even highly diluted blood elicits avoidance behavior in conspecifics.Citation21 This finding is relevant because spiny lobsters, like many decapods, can escape imminent death from predators by shedding limbs (autotomy), a process that causes non-lethal injuries.Citation22 Loss of blood through autotomy is limited but might benefit nearby conspecifics if they can detect it.Citation21 Interestingly, Parsons and Eggleston,Citation23 also using Y-mazes, found that P. argus lobsters significantly chose shelters with scents of intact conspecifics but responded at random to shelters emanating scents from lobsters that had been subjected to the breakage of three limbs before the trials.
Given these findings, I reanalyzed results from our trialsCitation1,Citation20 (including some not previously reported) because some of our experimental lobsters lost limbs as a result of handling just before the trials or during acclimatization. Thus, for each species, I separated all trials into three groups depending on whether the stimulus lobster was intact (stimulus A), non-lethally injured (i.e., had lost one or more limbs, stimulus B), or lethally injured (stimulus C). I separated the P. argus trials in this way irrespective of season, but further separated the P. guttatus trials into trials conducted in the spring or in late summer (but trials with stimuli C were conducted only in the spring). Then, for each stimulus, I subjected the results to a 1-tail binomial test (α = 0.05) where the null probability of choosing the shelter receiving the stimulus was equal to 0.5. For each species, I pooled trials with stimuli B irrespective of whether the loss of limbs occurred just before the trial of during acclimatization because, although the blood of spiny lobsters clots very fast, its clotting time tends to increase with stress, particularly in captive lobsters.Citation24–Citation26 Also, the frequencies of stimuli B that had lost 1, 2 or ≥3 limbs did not differ significantly (χ24 = 5.246, p = 0.263) between the three groups of trials with these stimuli (one for P. argus, two for P. guttatus). On average, 59% of all lobsters used as stimuli B (n = 109) lost only one limb, 23% lost two, and 18% three to five limbs.
Panulirus argus lobsters significantly chose shelters with stimulus A, showed a random response to shelters with stimulus B, and significantly avoided shelters with stimulus C (). The shelter choice by lobsters subjected to stimuli A and B was consistent with Parson and Eggleston'sCitation23 results despite the variable number of limbs lost by our stimuli B. These results suggest that P. argus lobsters are able to assess the strength of conspecific alarm odors and to make a decision as to whether or not to avoid the source of these alarm odors accordingly. These results are consistent with previous findings that this species is highly chemosensitiveCitation21,Citation27 and with the notion that avoiding conspecific alarm odors is a particularly effective antipredator strategy for highly gregarious species.Citation1,Citation3,Citation4
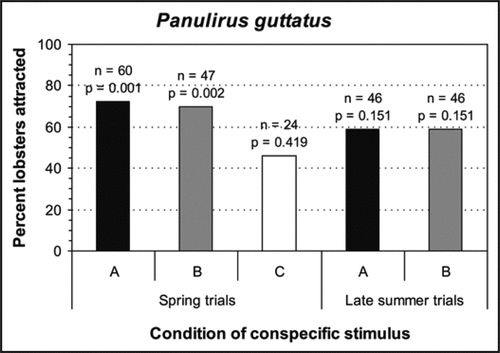
In contrast, the shelter choice by P. guttatus lobsters subjected to stimuli A or B was similar in the spring (attraction) and also in late summer (random), whereas the shelter choice of those subjected to stimuli C in the spring did not differ from random (). Thus, for these lobsters, the presence of weak conspecific alarm odors would not appear to affect the seasonal tendency to aggregate. In the season of high reproductive activity, some individuals would appear to avoid shelters with strong conspecific alarm odors (stimulus C), but the overall random response to this stimulus is consistent with the more cryptic antipredator behavior of this species. Therefore, although all species of spiny lobster likely use conspecific alarm odors for risk-assessment, the behavioral response of lobsters of each species to these cues would be that which reduces the risk of encountering active predators most.
Figures and Tables
Figure 1 Results of Y-maze experiments investigating the effects of scents emanating from intact (A), non-lethally injured (B) or lethally-injured (C) conspecifics on shelter choice by individuals of Panulirus argus. p-values were based on one-tail binomial tests to test for attraction, where null probability of choosing a shelter receiving scents emanating from the stimulus = 0.5.
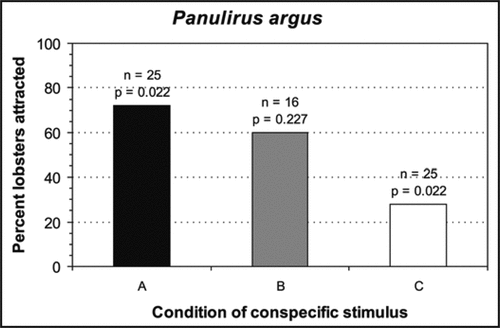
Figure 2 Results of Y-maze experiments investigating the effects of scents emanating from intact (A), non-lethally injured (B), or lethally-injured (C) conspecifics on shelter choice by individuals of Panulirus guttatus. The spring trials correspond to the season of high reproductive activity, and the late-summer trials correspond to the season of low reproductive activity. p-values were based on one-tail binomial tests to test for attraction, where null probability of choosing a shelter receiving scents emanating from the stimulus = 0.5.
Acknowledgements
I thank C. Barradas-Ortiz and F. Negrete-Soto for their invaluable technical support in both field and laboratory activities, and E. Ramírez-Zaldívar, A. Osorio-Arciniegas, R. Domínguez-Gallegos, M. Pérez-Ortiz, J. Valladárez-Cob, K. Baeza-Martínez and D. Placencia-Sánchez for additional assistance. Funding was provided by Consejo Nacional de Ciencia y Tecnología, México (Project 40159-Q). Permits to capture experimental lobsters were issued by Comisión Nacional de Acuacultura y Pesca, México.
Addendum to:
References
- Briones-Fourzán P, Ramírez-Zaldívar E, Lozano-Álvarez E. Influence of conspecific and heterospecific aggregation cues and alarm odors on shelter choice by syntopic spiny lobsters. Biol Bull 2008; 215:182 - 190
- Katz LB, Dill LM. The scent of death: chemosensory assessment of predation risk by prey animals. Ecoscience 1998; 5:361 - 368
- Wisenden BD. Olfactory assessment of predation risk in the aquatic environment. Philos Trans R Soc Lond B 2000; 355:1205 - 1208
- Dicke M, Grostal P. Chemical detection of natural enemies by arthropods: an ecological perspective. Annu Rev Ecol Syst 2001; 32:1 - 23
- Ratchford SG, Eggleston DB. Size- and scale-dependent chemical attraction contribute to an ontogenetic shift in sociality. Anim Behav 1998; 56:1027 - 1034
- Ratchford SG, Eggleston DB. Temporal shift in the presence of a chemical cue contributes to an ontogenetic shift in sociality. Anim Behav 2000; 59:793 - 799
- Butler MJ, McDiarmid AB, Booth JD. The cause and consequences of ontogenetic changes in social aggregations in New Zealand spiny lobster. Mar Ecol Prog Ser 1999; 188:179 - 191
- Horner AJ, Nickles SP, Weissburg MJ, Derby CD. Source and specificity of chemical cues mediating shelter preference of Caribbean spiny lobster (Panulirus argus). Biol Bull 2006; 211:128 - 139
- Childress MJ. Duffy JE, Thiel M. Comparative sociobiology of spiny lobsters. Evolutionary Ecology of Social and Sexual Systems: Crustaceans as Model Organisms 2007; Oxford Oxford University Press 271 - 293
- Herrnkind WF, Childress MJ, Lavalli K. Cooperative defence and other benefits among exposed spiny lobsters: inferences from group size and behaviour. Mar Freshw Res 2001; 52:1113 - 1124
- Briones-Fourzán P, Lozano-Álvarez E. Coexistence of congeneric spiny lobsters on coral reefs: differences in den sharing by conspecifics and its potential antipredator benefits. Coral Reefs 2008; 27:275 - 287
- Briones-Fourzán P, Pérez-Ortiz M, Lozano-Álvarez E. Defense mechanisms and anti-predator behavior in two sympatric species of spiny lobster, Panulirus argus and P. guttatus. Mar Biol 2006; 149:227 - 239
- Cox C, Hunt JH, Lyons WG, Davis GE. Nocturnal foraging of the Caribbean spiny lobster (Panulirus argus) on offshore reefs of Florida USA. Mar Freshw Res 1997; 48:671 - 679
- Childress MJ, Hernkind WF. The guide effect influence on the gregariousness of juvenile Caribbean spiny lobsters. Anim Behav 2001; 62:465 - 472
- Nevitt G, Pentcheff ND, Lohmann KJ, Zimmer RK. Den selection by the spiny lobster Panulirus argus: testing attraction to conspecific odors in the field. Mar Ecol Prog Ser 2000; 203:225 - 231
- Dolan T, Butler MJ. The adaptive value of aggregation among juvenile Caribbean spiny lobster: an evaluation using individual-base modeling. J Crustacean Biol 2006; 26:565 - 578
- Lozano-Álvarez E, Briones-Fourzán P, Osorio-Arciniegas A, Negrete-Soto F, Barradas-Ortíz C. Coexistence of congeneric spiny lobsters on coral reefs: differential use of shelter resources and vulnerability to predators. Coral Reefs 2007; 26:361 - 373
- Sharp WC, Hunt JH, Lyons WC. Life history of the spotted spiny lobster, Panulirus guttatus, an obligate reef-dweller. Mar Freshw Res 1997; 48:687 - 698
- Wynne S, Côté I. Effects of habitat quality and fishing on Caribbean spotted spiny lobster populations. J Appl Ecol 2007; 44:488 - 494
- Briones-Fourzán P, Lozano-Álvarez E. Seasonal variations in chemical response to conspecific scents in the spotted spiny lobster, Panulirus guttatus (Latreille). N Z J Mar Freshw Res 2005; 39:383 - 390
- Shabani S, Kamio M, Derby CD. Spiny lobsters detect conspecific blood-borne alarm cues exclusively through olfactory sensilla. J Exp Biol 2008; 211:2600 - 2608
- Juanes F, Smith LD. The ecological consequences of limb damage and loss in decapod crustaceans: a review and proposition. J Exp Mar Biol Ecol 1995; 193:197 - 223
- Parsons DM, Eggleston DB. Indirect effects of recreational fishing on behavior of the spiny lobster Panulirus argus. Mar Ecol Prog Ser 2005; 303:235 - 244
- Tait J. Types of crustacean blood coagulation. J Mar Biol Assoc UK 1911; 9:191 - 198
- Jussila J, McBride S, Jago J, Evans LH. Hemolymph clotting time as an indicator of stress in western rock lobster (Panulirus cygnus George). Aquaculture 2001; 199:185 - 193
- Morrison PR, Morrison KC. Bleeding and coagulation in some Bermudan crustacea. Biol Bull 1952; 103:395 - 406
- Derby CD. Learning from spiny lobsters about chemosensory coding of mixtures. Physiol Behav 2000; 69:203 - 209