Abstract
Drosophila embryogenesis begins with 13 rapid nuclear divisions within a common cytoplasm. These divisions produce ~6,000 nuclei that, during the next division cycle, become encased in plasma membrane (PM) and generate the primary embryonic epithelium in the process known as cellularization. Despite the absence of PM boundaries between syncytial nuclei, the secretory membrane system is organized in functionally compartmentalized units around individual nuclei.1 We have recently used in vivo fluorescence imaging to characterize the dynamics of proteins in the PM of the embryonic syncytium. These studies revealed that the PM is polarized already before cellularization. One PM region resides above individual nuclei and has apical-like features, while PM regions lateral to nuclei have basolateral characteristics. Optical highlighting experiments showed that membrane components do not exchange between PM regions that reside above adjacent nuclei. An intact F-actin network was shown to be important for both the PM apicobasal-like polarity and the diffusion barriers within the syncytial PM. Our findings, as well as their possible implications, are further discussed in this Addendum.
During early fly embryogenesis nuclei rapidly divide without accompanying cytokinesis, thus producing a large, multinucleate cell with ∼6,000 nuclei at the embryo cortex. Only then do nuclei become encased in PM to form polarized epithelial cells at the embryo periphery.Citation2 Spatially asymmetric cues that originate in oogenesis are elaborated in the syncytial embryo to produce morphogen gradients that operate during the time of the syncytial divisions.Citation3 How are organelles organized within a common volume and in the presence of thousands of nuclei? How do gene and protein products traffic? How do these events relate to the generation and maintenance of the operating gradients? Such questions addressing the subcellular architecture of the multinucleate embryo cell have not been systematically explored, and should provide insights into the subcellular regulation of early developmental events.
An earlier study of the organization of the exocytic pathway in the syncytial fly embryo showed that maternally-loaded secretory organelles (ER and Golgi) are compartmentalized around individual nuclei.Citation1 Protein products destined for the PM were found to traffic in a localized, polarized fashion, and not randomly across the embryo cortex. The compartmentalization of membrane biosynthetic organelles around individual syncytial nuclei raised the possibility that each nucleus could differentiate the composition of its associated plasma membrane. We recently used in vivo fluorescence imaging to probe diffusion and architecture in the seemingly continuous PM. The PM encasing the thousands of nuclei was found to be polarized with PM regions above nuclei bearing apical-like features and PM lateral to nuclei containing basolateral characteristics. Furthermore, PM components did not diffuse between PM regions that reside above adjacent nuclei.Citation4
These findings strongly suggest that the compartmentalized exocytic pathways could differentiate and maintain a discrete composition of the membrane-bound organelles and associated transport intermediates contained in each nucleus-associated cytoplasmic domain. There is an increasing body of literature that highlights the importance of local anchoring, generation and translation of mRNA transcripts in controlling protein localization.Citation5 The recent description of dozens of mRNA localization patterns in the multinucleate embryo emphasizes a major role for mRNA localization in nucleating localized cellular machineries (e.g., localized cytoskeletal remodeling) around individual syncytial nuclei.Citation6 Finally, nuclei arrive and divide in a space with already established spatial asymmetries: anterior-posterior, dorsal-ventral and terminal gradients operate, so that each nucleus and its associated cytoplasmic domain experience a distinct set of transcripts and signaling cascades. The combinatorial effect of all these processes implies that each nucleus-associated unit has and is able to maintain a unique composition in the absence of physical boundaries between adjacent nuclei ().
Such compartmentalized behavior of nuclear-associated domains essentially shows active “cell bodies” in action and questions the current cell theory. The concept of “energids” was introduced already in 1892 by Julius SachsCitation7 to describe a nucleus with an associated portion of cytoplasm (“sphere of influence”).Citation7,Citation8 This concept was further elaborated by Daniel Mazia and further refined by Baluska and colleagues to the “cell body” (nucleus and a complement of perinuclear microtubules) as the smallest autonomous and self-reproducing unit of eukaryotic life.Citation8,Citation9 What organizes individual “cell bodies” in the embryonic syncytium? UV irradiation or aphidicolin treatment before nuclei migration to the cortex, or a mutation in the gnu locus, led to the uncoupling of centrosome and nuclear divisions in the syncytium.Citation10–Citation12 In all cases, in the absence of nuclei at the surface, centrosomes alone were able to divide and reorganize microtubules, actin and spectrin networks, as well as plasma membrane in the syncytium. These findings emphasize a major role for centrosomes in organizing a “cell body” behavior. It would be interesting to probe the presence of PM asymmetries under conditions where centrosomes and not nuclei are present.
Another aspect related to the presence of cell bodies in the syncytium is the diffusion of cytosolic proteins. The diffusive behavior of injected dextrans, as well as injected GFP (our unpublished FCS measurements and FLIP experiments) suggest that “inert” molecules are able to explore the embryo cytoplasm in its whole volume.Citation13 However, the diffusion of endogenous cytosolic proteins has not systematically been addressed, and such experiments would give insights into the organization of the cortex and the “integrity” of and/or “interconnectivity” between adjacent cell bodies. Such experiments would further shed light on the formation and/or maintenance of morphogen gradients: e.g., “trapping” and short-range diffusion of Bicoid within a given Energid was recently proposed as an operating mechanism for the Bicoid gradient maintenance.Citation14
Finally, the fact that the PM is polarized before cellularization raises the question of when PM asymmetries first arise, in particular the generation of a distinct basolateral component. One interesting possibility is that this component arises from a subgroup of maternally loaded mRNAs, which when translated, will help organize the PM. One such transcript is Toll. There are Toll maternal transcripts before nuclear migration, however its translation and PM delivery increases as soon as nuclei arrive at the cortex and over the course of the syncytial cycles.Citation15 Toll is a homophilic adhesion molecule.Citation16 One possibility is that increasing concentration of Toll induces clustering of Toll molecules in the PM in such a way that an interlocked network of adhesive belts forms around individual Cell Bodies at the cortex. Such a structure would provide mechanic stability at the cortex, during a period when between 400 and 5,000 nuclei need to be stably positioned both during interphases and as they divide. At the same time, self-organizing adhesive belts would induce a subcortical differentiation of the cytoskeleton (e.g., recruitment of septins, polarity complexes, etc.,) that could lead to the associated PM region becoming “basolateral”. Testing the emergence of PM asymmetries in embryos where Toll lacks its extracellular domain (i.e., has no adhesive properties) might provide new insights into how PM polarity arises.
Figures and Tables
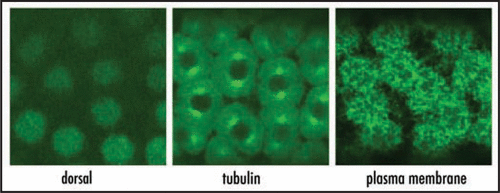
Figure 1 In the absence of plasma membrane boundaries between adjacent nuclei, cytoskeleton and plasma membrane are organized in a cell body like manner around individual nuclei (middle and right panel). Nuclei experience a different set of regulatory proteins, shown here in the nuclear gradient of the cell fate determinant, dorsal (left panel).
Addendum to:
References
- Frescas D, Mavrakis M, Lorenz H, Delotto R, Lippincott-Schwartz J. The secretory membrane system in the Drosophila syncytial blastoderm embryo exists as functionally compartmentalized units around individual nuclei. J Cell Biol 2006; 173:219 - 230
- Foe VE, Alberts BM. Studies of nuclear and cytoplasmic behaviour during the five mitotic cycles that precede gastrulation in Drosophila embryogenesis. J Cell Sci 1983; 61:31 - 70
- St Johnston D, Nusslein-Volhard C. The origin of pattern and polarity in the Drosophila embryo. Cell 1992; 68:201 - 219
- Mavrakis M, Rikhy R, Lippincott-Schwartz J. Plasma membrane polarity and compartmentalization are established before cellularization in the fly embryo. Dev Cell 2009; 16:93 - 104
- St Johnston D. Moving messages: the intracellular localization of mRNAs. Nat Rev Cell Dev Biol 2005; 6:363 - 375
- Lecuyer E, Yoshida H, Parthasarathy N, Alm C, Babak T, Cerovina T, et al. Global analysis of mRNA localization reveals a prominent role in organizing cellular architecture and function. Cell 2007; 131:174 - 187
- Sachs J. Beiträge zur Zellentheorie. Energiden und Zellen. Flora 1982; 75:57 - 67
- Baluška F, Volkmann D, Barlow PW. Baluška F, Volkmann D, Barlow PW. Cell-cell channels and their implications for Cell Theory. Cell-Cell Channels 2006; Georgetown and New York Landes Bioscience and Springer Verlag 1 - 18
- Baluška F, Volkmann D, Barlow PW. Eukaryotic cells and their cell bodies: Cell Theory revised. Ann Bot 2004; 94:9 - 32
- Yasuda GK, Baker J, Schubiger G. Independent roles of centrosomes and DNA in organizing the Drosophila cytoskeleton. Development 1991; 111:379 - 391
- Raff JW, Glover DM. Centrosomes, and not nuclei, initiate pole cell formation in Drosophila embryos. Cell 1989; 57:611 - 619
- Freeman M, Nusslein-Volhard C, Glover DM. The dissociation of nuclear and centrosomal division in gnu, a mutation causing giant nuclei in Drosophila. Cell 1986; 46:457 - 468
- Gregor T, Bialek W, de Ruyter van Steveninck RR, Tank DW, Wieschaus EF. Diffusion and scaling during early embryonic pattern formation. Proc Natl Acad Sci USA 2005; 102:18403 - 18407
- Lucchetta EM, Vincent ME, Ismagilov RF. A precise Bicoid gradient is nonessential during cycles 11–13 for precise patterning in the Drosophila blastoderm. PLoS ONE 2008; 3:3651
- Hashimoto C, Gerttula S, Anderson KV. Plasma membrane localization of the Toll protein in the syncytial Drosophila embryo: importance of transmembrane signaling for dorsal-ventral pattern formation. Development 1991; 111:1021 - 1028
- Keith FJ, Gay NJ. The Drosophila membrane receptor Toll can function to promote cellular adhesion. EMBO J 1990; 9:4299 - 4306