Abstract
The mature central nervous system has a very limited capacity to repair itself after injury or disease, often leading to lifelong disabilities. Two key questions in neurobiology are why the brain has such limited plasticity, and how we could enhance it. There has been extensive research on how external inhibitors, present in the mature central nervous system, cooperate to restrict neurite plasticity – the ability of neurons to sprout and reorganize their connections. In a recent article, we have described an unsuspected mechanism by which neurons control (and actually repress) their capacity to sprout in a cell-autonomous manner. Our discovery implies that protrusive potential is not lost in mature neurons but internally repressed. This discovery opens up new research avenues and has a strong potential from a translational standpoint. Here I review our previous results and propose a more general hypothesis on the molecular mechanisms controlling neurite plasticity.
The brain is a delicate organ that can be easily damaged and has a very limited capacity to repair itself. As the world population ages, neurodegeneration and neurotrauma are of great concern to society. Up until 20 years ago, it was thought that brain and spinal cord neurons just did not have the capacity to regenerate. Today we have exciting data that suggests the adult central nervous system (CNS) may be capable of self-repair if encouraged by physical rehabilitation. Facilitating this process, and understanding what the barriers to regeneration are, is today one of the therapeutic frontiers for the treatment of brain damage and disease.
The term “neurite plasticity” refers to neurons' ability to repair and reorganize their projections, or neurites. The decrease in neurite plasticity that accompanies CNS maturation is thought to result from a combination of both intrinsic neuronal changes and changes in the surrounding environment.Citation2,Citation3 A deficiency of growth factors, accompanied by the expression of inhibitory molecules for neurite growth by glial cells, means that the mature CNS is much less friendly for neurite plasticity than the embryonic or immature one.Citation2 At the same time, there is a decrease in neuronal morphological plasticity that accompanies CNS maturation and that seems to be inherent to the neuron.Citation3 Thus, regardless of the environment, mature neurons have limited capacity to sprout and create new connections.
In our recently published article,Citation1 we set up to identify the molecular basis of this decrease in neuronal morphological plasticity, with the ultimate goal of enhancing this capacity, or “rejuvenating” neurons, as a therapeutic strategy for brain disease. We envisioned that if neuronal morphology is controlled as in other cells,Citation4 there must be an internal repressor that prevents protrusion formation along the neurite shaft, which could eventually lead to repression of neurite plasticity in the mature CNS. In Mingorance-Le Meur and O'Connor, we validated such a hypothesis and identified a new pathway used by neurons to regulate their plasticity levels that provides novel targets for neurological diseases.Citation1
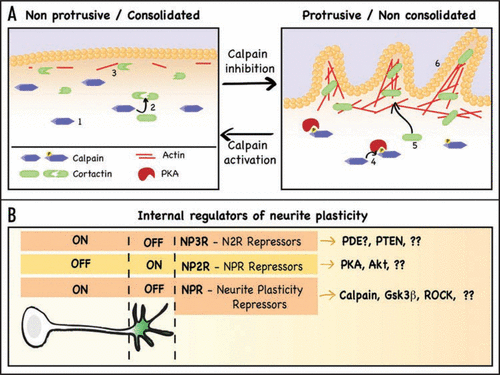
Using a combination of pharmacological and genetic manipulations of cultured neurons, we determined that neurite sprouting is linked to the process of neurite consolidation, the mechanism that generates the neurite as the growth cone advances.Citation5 Our research demonstrated that neurite consolidation requires active maintenance (repression of neurite sprouting) and that the strength of consolidation is responsible for neurite plasticity levels. We first identified that the protease calpain is a central player in the regulation of neurite plasticity by controlling consolidation (). Calpain activity is limited to the consolidated regions of the neuron (soma and neurite shaft) and inhibition of calpain suffices to promote neurite sprouting. We also showed that calpain limits protrusive activity by constantly degrading those proteins that are needed for neurite sprouting, such as cortactin (). The fact that these proteins are continuously being synthesized and degraded indicates that plasticity is not lost in mature neurons, as previously thought, but constantly repressed. Indeed, we also determined that those factors that promote neurite branching, such as neurotrophins, do so by inhibiting the pathway of neurite consolidation, that is, by derepressing neurite sprouting. We also demonstrated that pharmacological inhibition of calpain in vivo enhances neurite plasticity in the mature hippocampus, promoting sprouting and eventually leading to enhanced synaptogenesis. In some sense, these experiments validate the premise that it is possible to “rejuvenate” neurons by decreasing the strength of their consolidation.
We have therefore shown that neurite plasticity is linked to the process of neurite consolidation, and have also identified calpain as a key protein responsible for maintaining neurite consolidation by repressing neurite sprouting (and plasticity).Citation1 I hypothesize that this role is not exclusive of calpain but responds to a more general mechanism that separates the neuron in two domains (): one that allows protrusive activity (green) and one that actively represses it (white). In this model, calpain would be but one of the Neurite Plasticity Repressors (NPR), that are kept away from the growth cone by NPR Repressors (NP2R, PKA in the case of calpain). There is also evidence that suggests a third level, the NP3R, which antagonizes the NP2R along the neurite ().
In support of this theory on a more general system of repressors, at least two additional NPRs exist: ROCK and Gsk3β (). Both proteins are known to be involved in the repression of cellular protrusions, including neuronal growth cone collapse.Citation6,Citation7 In addition, inhibition of ROCK and Gsk3β has been shown to promote the formation of new filopodiaCitation8 or branchesCitation9 along the neurite shaft, which would position them in the role of NPRs ().
A key aspect of the NPRs' function is that their activity must largely be limited to the consolidated areas of the neuron, which is true at least for calpain and Gsk3β.Citation1,Citation10 A better understanding of the signaling that creates this spatial restriction in neurons is still needed, and would complete the picture of how the internal regulation of plasticity works. However, it is already known that Gsk3β is locally inhibited at the growth cone by Akt but “protected” at the neurite shaft by PTEN, a phosphatase that antagonizes PI3K signaling.Citation10 In the case of calpain, we have already shown that it is inhibited at the growth cone by PKA (its NP2R),Citation1 but the protein(s) playing the role of NP3R in this pathway is unknown. The protein most likely to antagonize PKA at the neurite shaft is a cAMP phosphodiesterase, and I speculate that one or more phosphodiesterases localize, or are preferentially active, along the neurite shaft, playing an equivalent role to that of PTEN as NP3Rs (). I envision that as we progress in our understanding of neurite plasticity signaling, the proposed three-layer multi- repressor pathway will emerge.
In summary, I believe our recent article provides a significant conceptual advance, revealing that neurite plasticity is internally regulated by the neuron and that protrusive potential is not lost in mature neurons but repressed. These findings, together with the proposed model of Neurite Plasticity Repressors, open up exciting new avenues for researching the molecular regulation of neurite plasticity and exploring how it is involved in normal and pathological brain function.
Figures and Tables
Figure 1 Summary of calpain regulation of neurite consolidation and proposed model of neurite plasticity regulation. (A) Summary of the signaling pathway described in Mingorance-Le Meur and O'Connor.Citation1 There are two morphological domains in neurons: a non protrusive one, comprised by soma and neurite shafts, and a protrusive one, comprised by growth cones and occasional regions of the shaft (such as branching points). The non protrusive regions (left) require active maintenance not to protrude, a process called consolidation. The protease calpain (1) is active in the consolidated regions, where it degrades proteins needed to promote actin polymerization, such as cortactin (2), thereby limiting the formation of actin patches (3), a necessary precursor for membrane protrusion. The protrusive regions (right) have high levels of cAMP and of active PKA, which phorphorylates and inhibits calpain (4) and allows the accumulation of cortactin (5), now free to associate with actin and promote the formation of actin patches and actin-rich protrusions (6). The transition between both domains is achieved by turning on or off the repressor controlling consolidation, in this case calpain. (B) Proposed model for the internal regulation of neurite plasticity. Neurite plasticity is linked to neurite consolidation, being higher when consolidation is weak, and decreasing as neurite consolidation becomes stronger. This means that neurite plasticity is directly repressed by those molecules that promote consolidation. I propose a model in which calpain is just one example of a Neurite Plasticity Repressors (NPR), proteins that repress the machinery necessary to create cell protrusions and that constitute a first level of repressors. These repressors would be kept away from the growth cone by NPR Repressors (NP2R), for example cAMP/PKA, creating a domain where the machinery of sprouting is not repressed. Activation of these NP2Rs at the neurite shaft would reduce consolidation and allow neurite sprouting. A third level of repressors might contribute to define the boundary between the shaft and the growth cone by antagonizing the NP2Rs along the neurite, thereby promoting NPRs activation. This would be the case of the phosphatase PTEN, and a potential phosphodiesterase that would help maintaining a limit a high/low cAMP at the growth cone/shaft boundary. Despite the many missing pieces, the conservation of signaling pathways among different cell systems, combined with some observations from the literature, strongly suggests this three-layer multi-repressor pathway is responsible for controlling neurite morphology and plasticity.
Acknowledgements
This work was supported by a long-term EMBO fellowship and a Michael Smith Foundation for Health Research postdoctoral trainee award.
Addendum to:
References
- Mingorance-Le Meur A, O'Connor TP. Neurite consolidation involves constant repression of protrusive activity. EMBO J 2009; 28:248 - 260
- Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci 2006; 7:617 - 627
- Spencer T, Filbin MT. A role for cAMP in regeneration of the adult mammalian CNS. J Anat 2004; 204:49 - 55
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, et al. Cell migration: integrating signals from front to back. Science 2003; 302:1704 - 1709
- Dent EW, Gertler FB. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron 2003; 40:209 - 227
- Mueller BK, Mack H, Teusch N. Rho kinase, a promising drug target for neurological disorders. Nat Rev Drug Discov 2005; 4:387 - 398
- Eickholt BJ, Walsh FS, Doherty P. An inactive pool of GSK-3 at the leading edge of growth cones is implicated in Semaphorin 3A signaling. J Cell Biol 2002; 157:211 - 217
- Loudon RP, Silver LD, Yee HF, Gallo G. RhoA-kinase and myosin II are required for the maintenance of growth cone polarity and guidance by nerve growth factor. J Neurobiol 2006; 66:847 - 867
- Jiang H, Guo W, Liang X, Rao Y. Both the establishment and the maintenance of neuronal polarity require active mechanisms: critical roles of GSK-3beta and its upstream regulators. Cell 2005; 120:123 - 135
- Chadborn NH, Ahmed AI, Holt MR, Prinjha R, Dunn GA, Jones GE, et al. PTEN couples Sema3A signalling to growth cone collapse. J Cell Sci 2006; 119:951 - 957