Abstract
The volvocine green algae are a model system for the evolution of multicellularity and cellular differentiation. A combination of molecular genetic and phylogenetic comparative approaches has resulted in a detailed picture of the transition from single cells to differentiated, multicellular organisms in this group. To be useful as a model system, the volvocine algae should provide information that is relevant to other groups. Here I discuss recent advances in understanding the origins of multicellularity and cellular differentiation in the volvocine algae and consider the implications for such transitions in general. Several general principles emerge that are relevant to the origins of major multicellular groups, such as animals, plants, fungi, and red and brown algae. First, if the lessons learned from the volvocine algae can be generalized to other origins of multicellularity, we should expect these transitions to be understandable as a series of small changes, each potentially adaptive in itself. In addition, cooperation, conflict and mediation of conflicts among cells are likely to have played central roles. Finally, we should expect the histories of these transitions to include parallel evolution of some traits, periods of relatively rapid change interspersed with long periods of stasis, and simpler forms coexisting with more complex forms for long periods of time as in the evolution of the volvocine algae.
Introduction
The transition from single cells to differentiated multicellular organisms has happened only a handful of times, but the consequences for life on Earth have been immense. Each of the major macroscopic groups had its origin in such a transition, and each has subsequently diversified into thousands to millions of species. Collectively, the multicellular red, green and brown algae, land plants, animals and fungi have transformed the planet, changing the atmosphere, creating new niches and generating selective pressures affecting each other's evolution as well as that of species in other lineages.
Each of the lineages enumerated above represents at least one independent origin of differentiated multicellularity,Citation1 and careful comparison of these replicate experiments has the potential to reveal general principles involved in this type of transition. Unfortunately, the passage of time and the ravages of extinction have obscured much of the evidence for the intermediate stages in these transitions. Although in the volvocine green algae, abundant evidence remains in the form of extant species with various mixtures of derived and ancestral traits. The ∼50 species in this group display a diverse range of body sizes and degrees of specialization, including single-celled forms and colonial forms with and without cellular differentiation. This diversity makes the volvocine algae a uniquely useful model system for understanding the evolution of multicellularity.
The Transition to Multicellularity in the Volvocine Algae
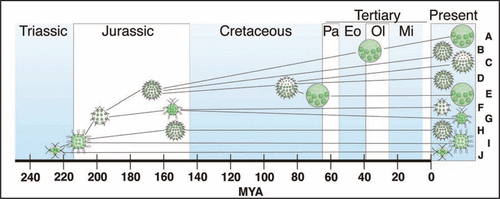
Phylogenetic comparative studies have generated a roadmap for understanding the evolution of differentiated multicellularity in the volvocine algae. The developmental changes involved in this transition have been identifiedCitation2 and reconstructed in a phylogenetic framework.Citation3 The emerging picture of volvocine evolution is complicated. Some changes had multiple, independent origins, and some characters reverted from derived back to ancestral statesCitation3 (). The pace of change has been erratic, with a relatively rapid series of changes taking place early on and relatively little change over long time spans in some lineages.Citation4 Some extant species are living fossils whose basic body plans have changed little in the last 200 million years.Citation4
Multicellular volvocine algae first appeared around 220 million years ago (MYA; 95% Bayesian credibility interval 223 ± 24 MYA)Citation4 (). In some Triassic pond or puddle, the offspring of a single-celled alga failed to separate after cytokinesis, remaining embedded in a glycoprotein extracellular matrix (ECM). The production of ECM had important consequences, as it bound the offspring of a given cell to a common fate. As a public good,Citation5,Citation6 or “commons,”Citation7 ECM required individual cells to invest resources for a shared benefit. This cooperative trait may have created the potential for conflict and cheating: individual cells that invested fewer resources into ECM production would have more resources remaining for reproduction.Citation3
Around the same time as the first multicellular forms appeared, determination of the number of daughters produced by a cell changed from environmental control to genetic control.Citation4 By limiting the benefit of defection, genetic control of cell number may have served as a mechanism to mediate conflicts among cells over ECM production.Citation3 Together, the key innovations of cooperation in the form of ECM production and conflict mediation in the form of genetic control of cell number set the stage for the relatively rapid series of integrative changes that followed.
Within a relatively short time after their divergence from unicellular ancestors, the ancestors of the Volvocaceae developed into highly integrated multicellular organisms. By the early Jurassic (200 ± 22 MYA) the developmental process of inversion established a spheroidal body plan with the cells arranged at the peripheryCitation4 (). This basic morphology has been retained in the modern genera Yamagishiella and Eudorina (Volvulina has a similar body plan but probably evolved a large volume of ECM independently).
In at least three separate lineages, a subset of cells specialized in motility, sacrificing their own reproduction.Citation3 Somatic specialization led to a partial division of labor, in which some cells contribute to both reproduction and motility and others only to motility. In a few species of Volvox, including V. carteri, the division of labor has subsequently become complete, with specialized reproductive cells that do not contribute to motility. In most cases the division of labor has persisted to the present; however, the history of these characters includes losses of both somatic and reproductive specialization.Citation3,Citation4 Unlike the other developmental changes mentioned here, it is still not known when most origins and losses of cellular differentiation occurred.Citation4
Although Volvox was first described by van Leeuwonhoek over 300 years ago,Citation8 volvocine species are still being described at a surprising rate.Citation9,Citation10 Cryptic species are common as well, as molecular phylogenies often show that a single species name masks two or more independent lineages.Citation3,Citation10–Citation14 There is good reason to hope that some newly discovered species could help to resolve some of the questions that remain about the order and timing of changes. An early diverging sister species to the known members of Volvox section Volvox (A in ), for example, could substantially narrow the uncertainty about when soma evolved in this lineage.
General Lessons for the Evolution of Multicellularity
The transition to multicellularity in the volvocine algae has been broken down into a series of small, potentially adaptive changes.Citation2,Citation3 Estimates of the timing of these changes make up the most complete and detailed timeline available for any such transition.Citation4 But how much of what we have learned about this transition can be generalized to other origins of differentiated multicellularity? As with any model organism, the volvocine algae are a compromise between tractability and relevance. Some of what we learn from them is likely to apply broadly to multicellular groups; other features of their evolution will be uncommon or unique to the volvocine algae themselves.
The transition from single cells to complex, differentiated multi- cellular organisms can seem an unbridgeable gap. The example of the volvocine algae shows that this gap can be crossed by gradual accumulation of small changes; the lack of data remaining from other such transitions should not be interpreted as evidence to the contrary. Rather, the shortage of intermediate forms in the major multicellular groups is likely a result of extinction of early diverging lineages that might have retained ancestral traits. Volvocine algae are still a relatively young group as multicellularity goes: roughly half the age of land plants,Citation15 a third the age of animalsCitation16 and a sixth that of red algae.Citation17 A few well-placed extinctions would have erased the evidence of the intermediate stages between single cells and integrated multicellular organisms, making the reconstruction of these steps impossible.
The importance of cooperation, conflict and conflict mediation in the early stages of the transition is likely a general principle for origins of multicellularity.Citation18–Citation20 The lower-level units in any such transition (the cells) must cooperate, and the ever-present temptation to defect must be suppressed if integrative traits such as cellular differentiation are to arise. The timing of changes in the volvocine algae suggests that cooperation in ECM production and conflict mediation in the form of genetic control of cell number were key innovations that set the stage for the relatively rapid sequence of integrative changes that followed. Genetic control of cell number is not a universal feature of differentiated multicellular organisms, though, and it remains to be seen what mechanisms of conflict mediation allowed the integration of early animals, plants, fungi and macroscopic algae.
Above all, the volvocine algae show that the transition to a new, higher level of complexity was not a constant, progressive process. The changes leading to a new kind of individual were erratic in time and inconsistent across lineages. Some changes happened more than once, others have reversed course and species with various levels of organization have coexisted across geological eras. We should not be surprised to find these complications in other transitions to differentiated multicellularity.
Given the contingent and stochastic nature of the evolutionary process, it is unlikely that the origins of the major multicellular groups were characterized by simple, progressive increases in complexity. Rather, the origins of animals, plants, fungi and macroscopic algae probably had much in common with that of the multicellular volvocine algae. If the complete stories of these transitions are ever fully reconstructed, we should expect them to include multiple independent origins of traits, periods of relatively rapid change interspersed with long periods of stasis, and simpler forms, now extinct, coexisting with more complex forms for long periods of time.
Figures and Tables
Figure 1 Youngest estimates of first appearance of volvocine body plansCitation4 showing relatively rapid, early diversification of body plans (Triassic—Jurassic) and relative stability in most lineages thereafter (Cretaceous—Present). As an example of multiple origins, somatic cells had three independent origins: in Astrephomene (H), Volvox section Volvox (A), and the clade including Pleodorina and the remaining species of Volvox (C–E). Examples of reversals to ancestral traits include the reduction in ECM volume in the ancestors of Pandorina (G) and the loss of soma in one lineage of Eudorina (D). In (A, C, E and H), the smaller cells are somatic, while the larger cells are reproductive. (A and E) Volvox, with hundreds to tens of thousands of small somatic cells, a few much larger reproductive cells, and a large volume of ECM; (B and D) Eudorina, with 16–32 undifferentiated cells and a large volume of ECM; (C) Pleodorina, with 64–128 cells, ∼10–50% of which are somatic (in the anterior), and a large volume of ECM; (F) Volvulina, with 8–16 undifferentiated cells and a large volume of ECM; (G) Pandorina, with 8–16 cells and a small volume of ECM; (H) Astrephomene, with 32–64 cells, ∼3–6% of which are somatic (in the posterior), and a large volume of ECM; (I) Gonium, with 8–32 undifferentiated cells arranged in a flat or slightly curved plate; (J) Basichlamys, with four undifferentiated cells embedded in a small volume of ECM.Citation21–Citation23 Pa, Paleocene; Eo, Eocene; Ol, Oligocene; Mi, Miocene; MYA, Million years ago.
References
- Bonner JT. The origins of multicellularity. Integr Biol 1998; 1:27 - 36
- Kirk DL. A twelve-step program for evolving multicellularity and a division of labor. Bioessays 2005; 27:299 - 310
- Herron MD, Michod RE. Evolution of complexity in the volvocine algae: transitions in individuality through Darwin's eye. Evolution 2008; 62:436 - 451
- Herron MD, Hackett JD, Aylward FO, Michod RE. Triassic origin and early radiation of multicellular volvocine algae. Proc Natl Acad Sci USA 2009; 106:3254 - 3258
- Samuelson PA. The pure theory of public expenditure. Rev Econ Stat 1954; 36:387 - 389
- Samuelson PA. Diagrammatic exposition of a theory of public expenditure. Rev Econ Stat 1955; 37:350 - 356
- Hardin G. The tragedy of the commons. Science 1968; 162:1243 - 1248
- van Leeuwonhoek A. Part of a letter from Mr Antony van Leeuwenhoek, concerning the worms in sheeps livers, gnats and animalcula in the excrements of frogs. Philos Trans Royal Soc London 1700; 22:509 - 518
- Nakazawa A, Krienitz L, Nozaki H. Taxonomy of the unicellular green algal genus Vitreochlamys (Volvocales), based on comparative morphology of cultured material. Euro J Phycol 2001; 36:113 - 128
- Nozaki H, Ott FD, Coleman AW. Morphology, molecular phylogeny and taxonomy of two new species of Pleodorina (Volvocaceae, Clorophyceae). J Phycol 2006; 42:1072 - 1080
- Coleman AW. Phylogenetic analysis of “Volvocacae” for comparative genetic studies. Proc Natl Acad Sci USA 1999; 96:13892 - 13897
- Nozaki H. Origin and evolution of the genera Pleodorina and Volvox (Volvocales). Biologia 2003; 58:425 - 431
- Nozaki H, Krienitz L. Morphology and phylogeny of Eudorina minodii (Chodat) Nozaki et Krienitz, comb. nov. (Volvocales, Chlorophyta) from Germany. Euro J Phycol 2001; 36:23 - 28
- Nozaki H, Misawa K, Kajita T, Kato M, Nohara S, Watanabe MM. Origin and evolution of the colonial Volvocales (Chlorophyceae) as inferred from multiple, chloroplast gene sequences. Mol Phylogenet Evol 2000; 17:256 - 268
- Kenrick P, Crane PR. The origin and early evolution of plants on land. Nature 1997; 389:33 - 39
- Love GD, Grosjean E, Stalvies C, Fike DA, Grotzinger JP, Bradley AS, et al. Fossil steroids record the appearance of Demospongiae during the Cryogenian period. Nature 2009; 457:718 - 721
- Butterfield N. Bangiomorpha pubescens n. gen., n. sp.: implications for the evolution of sex, multicellularity and the Mesoproterozoic/Neoproterozoic radiation of eukaryotes. Paleobiology 2000; 26:386 - 404
- Michod RE, Nedelcu A. Moya A, Font E. Cooperation and conflict during the unicellular-multicellular and prokaryotic-eukaryotic transitions. Evolution: from Molecules to Ecosystems 2003; Oxford Oxford University Press 195 - 208
- Michod RE, Roze D. Cooperation and conflict in the evolution of individuality III. Transitions in the unit of fitness. In: Nehaniv CL, ed. Mathematical and computational biology: computational morphogenesis, hierarchical complexity and digital evolution. Amer Math Soc 1999; 47 - 92
- Michod RE, Roze D. Cooperation and conflict in the evolution of multicellularity. Heredity 2001; 86:1 - 7
- Iyengar MOP, Desikachary TV. Volvocales 1981; New Delhi Indian Council of Agricultural Research
- Nozaki H. Morphology and taxonomy of two species of Astrephomene in Japan. J Japan Bot 1983; 58:345 - 352
- Nozaki H. Morphology and evolution of sexual reproduction in the Volvocaceae (Chlorophyta). J Plant Res 1996; 109:353 - 361