Abstract
Oviposition is evoked by plant compounds, which are recognized by chemoreceptive organs of insects. The swallowtail butterfly, Atrophaneura alcinous, oviposits its eggs on the host plant, Aristolochia debilis, in the presence of only two stimulating compounds: an alkaloid, aristolochic acid, and a monosaccharide, sequoyitol. In our previous study, a unique protein of 23 kDa [Oviposition stimulant(s) binding protein (OSBP)] was found in the forelegs of female, but not male, A. alcinous. The electrophysiological response of A. alcinous to an extract of A. debilis was depressed by the presence of OSBP antiserum, suggesting that OSBP presumably binds to oviposition stimulant(s). We show here, using a highly sensitive fluorescence micro-binding assay that native OSBP binds to a main oviposition stimulant, aristolochic acid, from its host plant, A. debilis. Three-dimensional molecular modeling studies also gave a reasonable structure for the OSBP / aristolochic acid complex. This is the first report of a native chemoreceptive protein binding to an oviposition stimulant ligand in insects.
An Oviposition Stimulant Binding Protein
Sensory reception plays very important roles in survival, especially for insects,Citation1–Citation7 and butterflies have evolved a specialized chemoreception system for laying eggs. For adult female butterfly, oviposition is an essential behavior for generating progeny. Therefore, it is necessary for the female butterflies to recognize their specific host plants. Chemoreceptive proteins in taste organs are thought to have important roles for the precise recognition of host plant compounds.Citation6,Citation7 The swallowtail butterfly, Atrophaneura alcinous, oviposits its eggs on the host plant, Aristolochia debilis, in the presence of only two triggering compounds: an alkaloid, aristolochic acid and a monosaccharide, sequoyitol. The oviposition behavior of butterflies is induced by recognition of the plant compounds via receptors in the tarsus of the foreleg. Using scanning electron microscopy, we observed tarsal contact chemosensilla in the foreleg; the female of A. alcinous has a toothbrush-like dense cluster of sensilla, which is larger than that of the male ().
Insect odorant-binding proteins (OBPs) are small, water-soluble proteins that are widely found in the olfactory systems of various species.Citation8–Citation10 OBPs are involved in the first specific biochemical step of odor reception and are thought to carry lipophilic odorants to the olfactory receptor cells through hydrophilic surroundings.Citation8–Citation16 The molecular cloning of insect OBPs is ongoing; however, few structural studies correlating function to ligand-binding activities have been reported.Citation17,Citation18
We have isolated a soluble protein of 23 kDa from A. alcinous.Citation19 Western blot analysis showed that this protein was expressed only in the female, and not in the male. Moreover, the protein is localized in the tarsi. We isolated this protein and determined the sequence of the N-terminal 23 amino acids. We then cloned its cDNA by RT-PCR and RACE. The deduced sequence of 212 amino acids is 38% homologous to a bilin-binding protein (BBP), of the cabbage butterfly, Pieris brassicae. Two consensus sequences from the lipocalin family of proteins were found in the sequence, suggesting that it is a binding protein for lipophilic ligands. This protein possibly plays an important role in the sensory process for oviposition, and is considered to be an oviposition stimulant(s) binding protein (OSBP).
Three-dimensional structure modeling of OSBP, based on the crystal structure of BBP, has suggested that aristolochic acid, an oviposition stimulant compound of A. alcinous, could bind OSBP.Citation20 Indeed, a binding assay, measuring fluorophore conjugated to a ligand molecule under an internal reflection fluorescence microscope, showed that OSBP binds to aristolochic acid.Citation21–Citation24
Immunohistochemistry
Localization of OSBP in male and female tarsi was investigated by immunohistochemistry using an anti-OSBP antiserum. Sections were counter-stained with Evans blue to reduce auto-fluorescence shown in red-orange. Strong auto-fluorescence was observed in tarsi, especially at the cuticle (). Green fluorescence in (without the anti-OSBP antiserum) is probably due to the non-specific binding of the secondary antibody. When sections were reacted with the anti-OSBP antiserum (), strong green signals were observed at the sensilla. OSBP is, therefore, likely to be localized at the sensilla of female tarsi.
Electrophysiological Responses of Chemosensilla
An electrophysiological study showed that the female tarsus was stimulated by a methanolic extract from the host plant, A. debilis ( and B). We investigated the responses to two main compounds of A. debilis, hydrophilic sequoyitol and lipophilic aristolochic acid. When sensilla were treated with aristolochic acid, one or two kinds of impulses were observed (). When sensilla were treated with sequoyitol, only one kind of impulse was observed (). When sensilla were stimulated by a methanolic extract from A. debilis, two to three different trains of impulses with differently sized amplitudes were usually observed. The sensilla were then pretreated with the antiserum raised against OSBP for ten minutes and then stimulated with the stimulating solution. The response was partially suppressed by the pretreatment (). The suppression by anti-OSBP antibody was removed by washing with water, suggesting that the binding of the antibody is reversible.Citation19 The results of the electrophysiological experiments suggest that OSBP plays the role of a binding receptor in the chemosensory signal transduction system for oviposition, probably as a carrier protein of the stimulants.
OSBP is present only in females of A. alcinous and can bind to aristolochic acid, a major oviposition stimulant of the host plant, A. debilis.Citation20 OSBP binds to aristolochic acid, suggesting that OSBP is involved in the chemosensory mechanism of A. alcinous. OSBP is, therefore, a candidate molecule for the transfer of aristolochic acid to receptors or for the activation of receptor molecules of the chemosensory neurons following ligand binding.
Figures and Tables
Figure 1 Scanning electron micrographs (SEM) of the ventral side of the 4th and 5th tarsi Atrophaneura alcinous. (A) Male and (B) female. Scale bar, 100 µm.
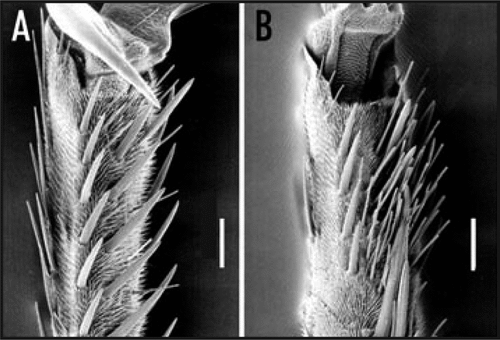
Figure 2 Immunohistochemical localization of OSBP in chemosensory organs of A. alcinous. (A) Female chemosensory organs without antiserum against OSBP. (B) Female chemosensory organs with antiserum against OSBP. Arrowheads indicate the cuticle. Green fluorescence indicates OSBPs signals, and auto-fluorescence is shown in orange. Scale bar, 20 µm.
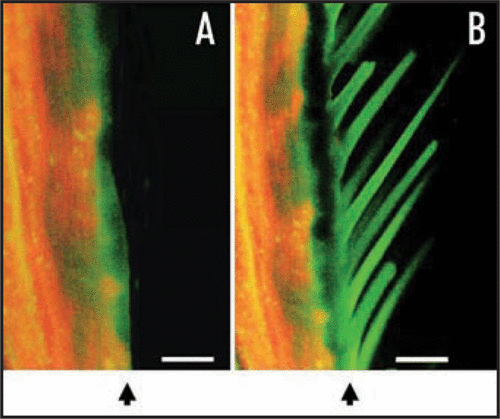
Figure 3 Electrophysiological responses to the plant compounds recorded from tarsal contact chemosensilla in a female A. alcinous. (A) Responses to control solution [containing 5% dimethyl sulfoxide (DMSO) and 50 mM choline chloride]. (B) Responses to the methanolic extracts of Aristolochia debilis. (C) Responses to aristolochic acid. (D) Responses to sequoyitol. (E) Responses to the methanolic extracts of A. debilis after ten minute treatment with antiserum against OSBP.
![Figure 3 Electrophysiological responses to the plant compounds recorded from tarsal contact chemosensilla in a female A. alcinous. (A) Responses to control solution [containing 5% dimethyl sulfoxide (DMSO) and 50 mM choline chloride]. (B) Responses to the methanolic extracts of Aristolochia debilis. (C) Responses to aristolochic acid. (D) Responses to sequoyitol. (E) Responses to the methanolic extracts of A. debilis after ten minute treatment with antiserum against OSBP.](/cms/asset/66653084-84ca-41c1-bd50-e9aedb6a5702/kcib_a_10908613_f0003.gif)
Acknowledgements
We would like to thank T. Wazawa, X.G. Zheng, M. Ishiguro, K. Yoshihara for their collaborations. We would like to thank Drs. Y. Ishii and T. Takanashi for their useful comments. We also thank Drs. R. Nishida, A. Yamanaka and Mr. Y. Tsuchihara for material supplies.
Addendum to:
References
- Awmack CS, Leather SR. Host plant quality and fecundity in herbivorous insects. Annu Rev Entomol 2002; 47:817 - 844
- Simmonds MS. Importance of flavonoids in insect-plant interactions: feeding and oviposition. Phytochemistry 2001; 56:245 - 252
- Bruce TJ, Wadhams LJ, Woodcock CM. Insect host location: a volatile situation. Trends Plant Sci 2005; 10:269 - 274
- Hallem EA, Dahanukar A, Carlson JR. Insect odor and taste receptors. Annu Rev Entomol 2006; 51:113 - 135
- Rausher MD. Search image for leaf shape in a butterfly. Science 1978; 200:1071 - 1073
- Nishida R, Fukami H. Oviposition stimulants of an aristolochiaceae-feeding swallowtail butterfly, Atrophaneura alicinous. J Chem Ecol 1989; 15:2565 - 2575
- Ohsugi T, Nishida R, Fukami H. Multi-component system of oviposition stimulants for a Rutaceae-feeding swallowtail butterfly, Papilio xuthus (Lepidoptera: Papilionidae). Appl Entomol Zool 1991; 26:29 - 40
- Vogt RG. Goldsmith MR, Wilkins AS. Molecular genetics of moth olfaction: a model for cellular identity and temporal assembly of the nervous system. Molecular Model Systems in the Lepidoptera 1995; Cambridge, UK Cambridge University Press 341 - 367
- Pelosi P. Perireceptor events in olfaction. J Neurobiol 1996; 30:3 - 19
- Vogt RG, Callahan ME, Rogers ME, Dickens JC. Odorant binding protein diversity and distribution among the insect orders, as indicated by LAP an OBP-related protein of the true bug Lygus lineolaris (Hemiptera, Heteroptera). Chem Senses 1999; 24:481 - 495
- Vogt RG, Riddiford LM, Prestwich GD. Kinetic properties of pheromone degrading enzyme: the sensillar esterase of Antheraea polyphemus. Proc Natl Acad Sci USA 1985; 82:8827 - 8831
- Prestwich GD, Du G, Laforest S. How is pheromone specificity encoded in proteins?. Chem Senses 1995; 20:461 - 469
- Steinbrecht RA. Are odorant-binding proteins involved in odorant discrimination?. Chem Senses 1996; 21:719 - 727
- Steinbrecht RA. Eguchi E, Tominaga S. Olfactory receptors. Atlas of Arthropod Sensory Receptors 1999; Tokyo, Japan Springer-Verlag 155 - 176
- Ziegelberger G. The multiple role of the pheromone-binding protein in olfactory transduction. Olfaction in mosquito-host interactions. CIBA Found Symp 1996; 200:267 - 280
- Kaissling KE. Pheromone deactivation catalyzed by receptor molecules: a quantitative kinetic model. Chem Senses 1998; 23:385 - 395
- Campanacci V, Krieger J, Bette S, Sturgis JN, Lartigue A, Cambillau C, et al. Revisiting the specificity of Mamestra brassicae and Antheraea polyphemus pheromone-binding protein with a flurescence binding assay. J Biol Chem 2002; 276:20078 - 20084
- Ban L, Scaloni A, Ambrosio CD, Zhang L, Yahn Y, Pelosi P. Biochemical characterization and bacterial expression of an odorant-binding protein from Locusta migratoria. Cell Mol Life Sci 2003; 60:390 - 400
- Tsuchihara K, Ueno K, Yamanaka A, Isono K, Endo K, Nishida R, et al. A putative binding protein for lipophilic substances related to butterfly oviposition. FEBS Lett 2000; 478:299 - 303
- Tsuchihara K, Wazawa T, Ishii Y, Yanagida T, Nishida R, Zheng XG, et al. Characterization of chemoreceptive protein binding to an oviposition stimulant using a fluorescent microbinding assay in a butterfly. FEBS Lett 2009; 583:345 - 349
- Funatsu T, Harada Y, Tokunaga M, Saito K, Yanagida T. Imaging of single fluorescent molecules and individual ATP turnovers by single myosin molecules in aqueous solution. Nature 1995; 374:555 - 559
- Wazawa T, Ueda M. Total internal reflection fluorescence microscopy in single molecule nanobioscience. Adv Biochem Eng Biotechnol 2005; 95:77 - 106
- Miyamoto Y, Muto E, Mashimo T, Iwane AH, Yoshiya I, Yanagida T. Direct inhibition of microtubule-based kinesin motility by local anesthetics. Biophys J 2000; 78:940 - 949
- Wazawa T, Ishii Y, Funatsu T, Yanagida T. Spectral fluctuation of a single fluorophore conjugated to a protein molecule. Biophys J 2000; 78:1561 - 1569