Abstract
Caveolae are small plasma membrane-associated invaginations that are enriched in proteins of the caveolin family in addition to, sphingolipids, glycosphingolipids and cholesterol. Caveolae have been implicated in several endocytic and trafficking mechanisms. Mutations in caveolins have been shown to cause disease and caveolae offer one site for pathogen entry. The Caenorhabditis elegans genome encodes two caveolins (cav-1 and cav-2); we have shown that these two proteins have distinct expression patterns. CAV-1 is found in the majority of cells in embryos and in the body-wall muscles, neurons and germ line of adult worms. CAV-2 is expressed in the intestine and is required for apical lipid trafficking. In the course of our studies, we generated several constructs to over-express caveolins in C. elegans. Here we show that over-expression of cav-1 protects against the decrease in brood size associated with the effects of heat shock and the presence of extrachromosomal arrays in heat-shocked animals. Furthermore, we show that over-expression of cav-2 in the nervous system increases the rate of egg-laying and total number of eggs laid.
Cells utilize a range of different mechanisms to mediate trafficking between the plasma membrane and intracellular membranes. One such mechanism involves caveolae, which are small (50–100 nm) invaginations in the plasma membrane. These invaginations are unusually rich in caveolin, sphingolipids, glycosphingolipids, cholesterol and many signaling proteins.Citation1,Citation2 Caveolae require caveolin for their formation and most caveolin appears to traffic to the plasma membrane; however, we and others have identified intracellular caveolin-containing bodies.Citation2–Citation4 Caveolae have been implicated in the endocytosis of a range of molecules and pathogensCitation2,Citation5,Citation6 and have also been implicated in a number of signaling processes.Citation2 Mammals encode three caveolin proteins; caveolin-1 is widely expressed in many tissue types, the closely related caveolin-3 protein is restricted to myocytes, and caveolin-2 has been shown to require caveolin-1 for transport to the plasma membrane.Citation2 Caveolins have been implicated in a wide range of processes, such as: cancer, lung disease, lipid homeostasis and disease, liver regeneration and dystrophies.Citation2,Citation7–Citation16
We have used Caenorhabditis elegans as a model system to explore the roles of caveolins. C. elegans encodes two caveolin proteins.Citation17 The C. elegans caveolin-2 protein (CAV-2) is localized to the apical membrane of intestinal cells. Ablation of cav-2 induces abnormal trafficking of yolk proteins and uptake of lipid markers; furthermore, cav-2 mutants suppress an intestinal phenotype induced by defective basolateral recycling in rme-1 and rab-10 mutants.Citation3 In contrast, caveolin-1 (cav-1) is widely expressed in eggs, and in many tissues at early larval stages but with maturation, CAV-1 becomes restricted to the germ line, nervous system, body-wall muscles, and most likely, the post-synaptic side of the neuromuscular junction.Citation18,Citation19 Ablation of CAV-1 function, using dominant-negative constructs based on dystrophic mutations identified in humans, induces neurotransmission and locomotion defects that may offer insights into the pathology of certain muscular dystrophies.Citation18
Overexpression of Caveolin-1 Protects Against Reduced Fecundity Caused by Extrachromosomal Arrays and Heat Shock
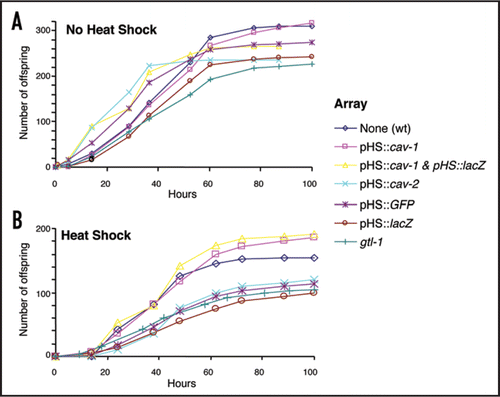
We found that overexpression of caveolin proteins was able to protect against deleterious effects of transgenes and heat shock in worms. In experiments designed to identify the effects of caveolin overexpression we used a heat-shock inducible promoter, hsp16-2 (pHS),Citation20 which drives gene expression in a wide variety of tissues, to express the caveolin genes. pHS::cav constructs were introduced into C. elegans using standard microinjection techniques, which result in transgenes which are present on extrachromosomal arrays (ECAs).Citation21 To test the efficiency of the pHS, we made pHS::cav-1::gfp and pHS::cav-2::gfp constructs. Worms incubated at 20°C had little or no detectible GFP, as measured by confocal microscopy; however, following a heat-shock of 33°C for 1 hour, we could detect GFP in most, if not all, tissues. We then used pHS::cav-1 and pHS::cav-2 constructs and a range of controls in heat shock experiments. In order to induce a high level of expression from the pHS::cav-1 and pHS::cav-2 animals, we heat-shocked animals twice daily for 1 hour at 33°C. The results in show that the various transgenes had little effect on brood size in untreated animals. However in heat shocked animals () we identified three effects. Firstly, the brood size of all animals is reduced compared to the untreated animals, which probably results from the temperature regimen that is likely to have many physiological effects. Secondly, in general, carrying an extrachromosomal array, for example the pHS::GFP, pHS::lacZ, pHS::cav-2 or gtl-1 (an array carrying a TRPM type channel geneCitation22 but no heat shock promoter) arrays, further reduces brood size. This suggests that the presence of transgenic arrays has a generic negative affect on fecundity. Thirdly, animals containing arrays that include pHS::cav-1 exhibit brood sizes, which are higher than that of both the other array containing lines and the wild type animals in this environment (). This suggests that the presence of cav-1 is protective against the deleterious effect of ECA in heat-shocked animals. To confirm that pHS::cav-1 was able to overcome the effects of any transgenic array we made animals carrying an array of both pHS::cav-1 and pHS::lacZ as the latter has a low brood size when expressed alone. Again the fecundity is restored. We used an un-paired t-test to compare the total brood-size of worms carrying both the pHS::cav-1 and pHS::lacZ constructs with worms carrying only the pHS::lacZ. Comparing strains in a non heat-shocked environment gave a p = 0.223 value; however, comparing worms from a heat-shocked environment gave a p = 0.003 value. Thus the presence of cav-1 appears to protect animals against the deleterious effects of transgenes in heat shocked animals. Further, the presence of pHS::cav-1 may even be able to partially compensate for the reduced brood size caused by heat shock. The mechanism of this effect is unclear. It has been shown that CAV-1 is highly dynamic in the germ line of C. elegans,Citation4 a possible site of action. However ECAs are generally silenced in the germ line. It is unclear whether the effect results from increased levels of CAV-1 in tissues which normally express the CAV-1 protein or , is due to ectopic expression. It has recently been suggested that C. elegans CAV-1 is unlikely to form caveolae,Citation23 thus making its role in this and other processes in C. elegans enigmatic.
Overexpression of Caveolin-2 Causes Increased Egg Laying
In a related set of experiments we overexpressed cav-1 and cav-2 in the nervous system of worms. We reasoned that overexpression of cav-1 in the nervous system, one of the main tissues expressing cav-1, might induce phenotypes indicative of its neuronal function and related to those we observed in animals carrying cav-1 dominant-negative constructs.Citation18 To this end, we cloned the cav-1 gene downstream from the neuron-specific promoter from the unc-119 gene.Citation24 To ensure that cav-1 was being expressed in neurons, we also made a cav-1::gfp construct. As a control, we made the same constructs previously described, but used the cav-2 gene. Surprisingly, we found no obvious phenotypes in animals overexpressing cav-1; however, we found that cav-2 overexpression induced an increase in the total number of eggs laid, and an increase in the rate of egg laying (data not shown). This phenotype was most obvious at 50 hours after the initiation of egg laying (cav-1 overexpression, mean = 173, sd = 18; cav-2 overexpression, mean = 307, sd = 37; N2 wild-type, mean = 174, sd = 14). Thus overexpression of cav-2 from the unc-119 promoter appears to improve fecundity in worms.
Although cav-1 is not solely expressed in neurons, it was surprising that its overexpression there produced no detectable, neuronally-controlled, phenotypes. In contrast, overexpression of cav-2, a gene expressed in the intestine, did generate a phenotype when overexpressed in the nervous system. Egg laying in C. elegans is under neuronal control thus changes in egg laying may be the result of changes in neuronal function brought about by the presence of ectopic CAV-2 protein. The ability of CAV-2 to form caveolae has not been tested;Citation23 however, bioinformatic analysis suggests that it is more likely to form caveolae than CAV-1.Citation3 Thus, CAV-2 may alter neuronal function by changing the trafficking in neurons or by changing the behavior of the membrane. The ability of cav-2 to alter neuronal function in contrast to cav-1 and the differential effects of cav-1 and cav-2 following heat shock further support the notion that the two proteins play different roles in cell function in C. elegans.
Figures and Tables
Figure 1 Overexpression of CAV-1 improves fecundity in heat-shocked worms carrying extrachromosomal arrays. Individual L4 stage worms were placed onto plates and allowed to lay eggs. N = 10 for each condition. Worms were transferred every 12 hours and the number of offspring on each plate was determined. (A) Shows the brood size of animals that received no heat-shock treatment, assayed at 20°C and (B) shows brood sizes for worms that were heat-shocked for 1 hour, twice daily, at 33°C. N2 indicates wild-type and pHS indicates the promoter from the heat-shock gene, hsp-16-2.
Acknowledgements
We are grateful to Andrew Fire for plasmids. Some C. elegans strains used in this work were obtained from the Caenorhabditis Genetic Center. This work was supported by the BBSRC and the MRC.
Addendum to:
References
- Lajoie P, Nabi IR. Regulation of raft-dependent endocytosis. J Cell Mol Med 2007; 11:644 - 653
- Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol 2007; 8:185 - 194
- Parker S, Walker DS, Ly S, Baylis HA. Caveolin-2 is required for apical lipid trafficking and suppresses basolateral recycling defects in the intestine of Caenorhabditis elegans. Mol Biol Cell 2009; 20:1763 - 1771
- Sato K, Sato M, Audhya A, Oegema K, Schweinsberg P, Grant BD. Dynamic regulation of caveolin-1 trafficking in the germ line and embryo of Caenorhabditis elegans. Mol Biol Cell 2006; 17:3085 - 3094
- Marsh M, Helenius A. Virus entry: open sesame. Cell 2006; 124:729 - 740
- Parton RG, Richards AA. Lipid rafts and caveolae as portals for endocytosis: new insights and common mechanisms. Traffic 2003; 4:724 - 738
- Cao H, Alston L, Ruschman J, Hegele RA. Heterozygous CAV1 frameshift mutations (MIM 601047) in patients with atypical partial lipodystrophy and hypertriglyceridemia. Lipids Health Dis 2008; 7:3
- Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev 2004; 84:1341 - 1379
- Dowling JJ, Gibbs EM, Feldman EL. Membrane traffic and muscle: lessons from human disease. Traffic 2008; 9:1035 - 1043
- Fernandez MA, Albor C, Ingelmo-Torres M, Nixon SJ, Ferguson C, Kurzchalia T, et al. Caveolin-1 is essential for liver regeneration. Science 2006; 313:1628 - 1632
- Frank PG, Lisanti MP. Caveolin-1 and liver regeneration: role in proliferation and lipogenesis. Cell Cycle 2007; 6:115 - 116
- Garg A, Agarwal AK. Caveolin-1: A new locus for human lipodystrophy. J Clin Endocrinol Metabol 2008; 93:1183 - 1185
- Goetz JG, Lajoie P, Wiseman SM, Nabi IR. Caveolin-1 in tumor progression: the good, the bad and the ugly. Canc Met Rev 2008; 27:715 - 735
- Heimerl S, Liebisch G, Le Lay S, Bottcher A, Wiesner P, Lindtner S, et al. Caveolin-1 deficiency alters plasma lipid and lipoprotein profiles in mice. Biochem Biophys Res Commun 2008; 367:826 - 833
- Kim CA, Delepine M, Boutet E, El Mourabit H, Le Lay S, Meier M, et al. Association of a homozygous nonsense caveolin-1 mutation with Berardinelli-Seip congenital lipodystrophy. J Clin Endocrinol Metab 2008; 93:1129 - 1134
- Williams TM, Lisanti MP. The Caveolin genes: from cell biology to medicine. Ann Med 2004; 36:584 - 595
- Tang Z, Okamoto T, Boontrakulpoontawee P, Katada T, Otsuka AJ, Lisanti MP. Identification, sequence and expression of an invertebrate caveolin gene family from the nematode Caenorhabditis elegans. Implications for the molecular evolution of mammalian caveolin genes. J Biol Chem 1997; 272:2437 - 2445
- Parker S, Peterkin HS, Baylis HA. Muscular dystrophy associated mutations in caveolin-1 induce neurotransmission and locomotion defects in Caenorhabditis elegans. Invert Neurosci 2007; 7:157 - 164
- Scheel J, Srinivasan J, Honnert U, Henske A, Kurzchalia TV. Involvement of caveolin-1 in meiotic cell cycle progression in Caenorhabditis elegans. Nat Cell Biol 1999; 1:127 - 129
- Candido EP, Jones D, Dixon DK, Graham RW, Russnak RH, Kay RJ. Structure, organization and expression of the 16-kDa heat shock gene family of Caenorhabditis elegans. Genome 1989; 31:690 - 697
- Mello C, Fire A. DNA transformation. Methods Cell Biol 1995; 48:451 - 482
- Kwan CS, Vazquez-Manrique RP, Ly S, Goyal K, Baylis HA. TRPM channels are required for rhythmicity in the ultradian defecation rhythm of C. elegans. BMC Physiol 2008; 8:11
- Kirkham M, Nixon SJ, Howes MT, Abi-Rached L, Wakeham DE, Hanzal-Bayer M, et al. Evolutionary analysis and molecular dissection of caveola biogenesis. J Cell Sci 2008; 121:2075 - 2086
- Maduro M, Pilgrim D. Identification and cloning of unc-119, a gene expressed in the Caenorhabditis elegans nervous system. Genetics 1995; 141:977 - 988