Abstract
Many insects deposit marking pheromones following egg-laying that signal an occupied and thus sub-optimal resource. Herbivorous insects mark host fruit or other vegetative plant parts after depositing eggs, while insect parasitoids deposit such pheromones directly on the cuticle of a particular life stage of their prey. These oviposition marking pheromones (OMPs) are then recognized by conspecifics, who avoid subsequent egg-laying in the previously utilized and unsuitable host. Since many host resources are capable of supporting a limited number of offspring, these pheromones function to decrease competition among the brood, which increases survival rate of the subsequent generation. In rare instances, distinct species of phytophagous and parasitic insects will inspect the same substrate following egg-laying.1 Recently, Stelinski et al.1 have demonstrated that in such instances, the herbivore is able to learn to recognize its predator’s OMP and utilize it to its advantage by avoiding oviposition into unsuitable host fruit. This recognition of a foreign marking pheromone occurs in a multitrophic context since both herbivore and parasitoid inspect, oviposit into, and mark the same substrate (e.i. fruit surface). In this Article Adendum, we further show that this recognition of a foreign pheromone is both context dependent and mediated by preimaginal conditioning.
Infochemicals mediate behavioral and physiological interactions among organisms. Intraspecific infochemicals are termed pheromones and can modify behaviors such as mate location, mating success, egg laying, aggregation and developmental processes. Allelochemicals facilitate interactions between species and have been broadly characterized as allomones, kairomones and synomones.Citation2 The ecology of infochemical production and use between organisms at different trophic levels (plant, herbivore and natural enemy) has been summarized,Citation3 primarily from the perspective of host location by a natural enemy. From an evolutionary perspective, the specificity, reliability and detectability of the signal should vary with host range of the herbivore as well as that of the natural enemy with monophagous and stenophagous organisms demonstrating the most specificity, although further comparative studies are needed.Citation3 Oviposition marking pheromones (OMPs) are deposited by many parasitic and phytophagous insects immediately following egg-laying. They function to modify the oviposition behavior of conspecifics such that subsequent eggs are not deposited into an already utilized resource.Citation4 These pheromones are recognized by tarsal and mouthpart receptors of gravid females inspecting potential oviposition sites.Citation4 The resulting effect is reduced time spent on the marked and previously utilized resource as well as reduced probability of oviposition.Citation4,Citation5 These signals likely evolved as a mechanism of avoiding superparasitism, reducing competition for limited host resources (plant or animal) among brood of conspecific organisms. The benefits of producing and recognizing these signals are dependent on the fitness gain of the marker and the receiver. Females that mark fruit are protecting their reproductive investment from conspecifics and also avoiding multiple self-ovipositions into the same fruit. Recognition of the signal by conspecifics benefits them because they avoid a food resource that is already occupied and that would reduce survival of their offspring.Citation6 This further refines host selection over use of kairomones alone.
Recently, Stelinski et al.Citation1 demonstrated that a fly species (Rhagoletis mendax Curran) recognizes and avoids ovipositing into fruit that was previously marked by the OMP deposited by its wasp parasitoid [Diachasma alloeum (Muesebeck)]. In this example, both the herbivorous insect host and its predator, in the form of a wasp parasitoid, examine, lay an egg into, and subsequently mark the same fruit surface, because ultimately both herbivore and parasitoid develop within the fruiting body of the same plant species. Although evidence for recognition and use of a foreign OMP has been reported previously for a related fly-parasitoid system,Citation6 the interaction between R. mendax and D. alloeum is perhaps the most unambiguous case presented to date.Citation1 Most OMPs described to date mediate interactions between female conspecifics,Citation4 and thus this unique example demonstrates that an infochemical synthesized and deposited by one species can be recognized and exploited by another occupying a different taxonomic order and trophic level and that this interaction requires learning.Citation1
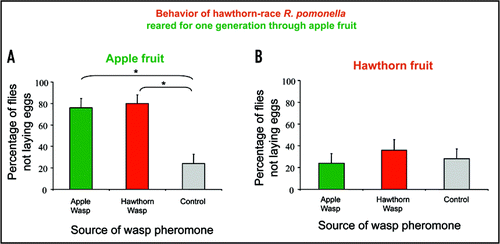
Herein, we further show that recognition of a foreign OMP is both context dependent and requires preimaginal conditioning. In addition to attacking R. mendax flies occurring on blueberry fruit, D. alloeum specifically parasitizes apple maggot flies, R. pomonella (Walsh).Citation7,Citation8 There are two host races within the R. pomonella species that are also ecologically separated by preferentially parasitizing either hawthorn (Crataegus mollis Scheele) or apple (Malus domestica Borkhausen) fruit.Citation9 These genetically-unique host races are considered an incipient stage of species formation separated pre-zygotically by odor-mediated attraction to the unabcised fruit of host plants, which constitute the exclusive site of larval development.Citation10–Citation12 Herein, we demonstrate that apple-race R. pomonella, having been reared through their natal apple fruit host, recognized and avoided both apple and hawthorn-race wasp OMPs on apple fruit, but did not respond to either wasp pheromone when it was presented to the flies on hawthorn fruit ( and B; See Stelinski et al.Citation1 for methods on insect rearing and behavioral experiments). Correspondingly, hawthorn-race R. pomonella, that were reared through their natal hawthorn fruit, recognized and avoided the OMPs of both hawthorn and apple-race wasps when encountering them on hawthorn fruit, but did not respond to these pheromones when ovipositing on apples ( and D). However, after artificially rearing hawthorn-race R. pomonella through apple fruit for only one generation, these flies recognized the OMPs of both hawthorn and apple-race wasps only on apple fruit, but not on their natal host, hawthorn fruit ( and B). These results demonstrate that one generation of rearing through a non-natal host fruit resource determined the response of adult flies to a foreign OMP. Preimaginal learning has been recently shown to shape the response of wasp parasitoids to the odors of their hosts.Citation13,Citation14 However, to our knowledge this is the first example showing that preimaginal conditioning modifies the behavior of an insect herbivore in response to its predator's OMP. Furthermore, this preimaginal learning appears to be independent of the peripheral nervous system since the antennae of hawthorn-race R. pomonella reared though both natal hawthorn and non-natal apple fruit were equally sensitive to their specific blend of hawthorn host plant volatilesCitation15 as measured by the electroantennogram techniqueCitation16 (Data not shown).
The host-marking pheromones of tephritid flies and other species that convey intraspecific information about a previously utilized oviposition resource have been termed ‘epideictic pheromones.’Citation17 In the currently described example, a host-marking pheromone produced by a wasp parasitoid species functions both to signal the parasitoid of a previously attacked hostCitation18 as well as the host fly of an unacceptable oviposition site. Because this pheromone functions interspecifically between distantly related organisms occupying different trophic levels, we would like to propose the term ‘xenodeictic’ to describe this effect.
Abbreviation
| OMP | = | oviposition marking pheromone |
Figures and Tables
Figure 1 Left: Percentage of apple-race Rhagoletis pomonella, reared through apple fruit, not depositing eggs into apple (A) or hawthorn (B) fruit treated with the oviposition marking pheromone of either apple- or hawthorn-race Diachasma alloeum. Right panels: Percentage of hawthorn-race Rhagoletis pomonella, reared through hawthorn fruit, not depositing eggs into hawthorn (C) or apple (D) fruit treated with the oviposition marking pheromone of either apple- or hawthorn-race Diachasma alloeum. Asterisks indicate significant differences among mean proportions as determined by logistic regression analysis (p < 0.05).
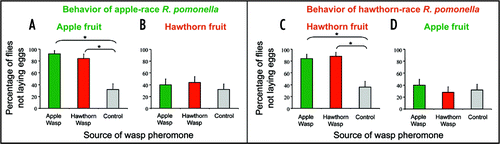
Figure 2 Percentage of hawthorn-race Rhagoletis pomonella, reared for one generation through apple fruit, not depositing eggs into apple (A) or hawthorn (B) fruit treated with the oviposition marking pheromone of either apple- or hawthorn-race Diachasma alloeum. Asterisks indicate significant differences among mean proportions as determined by logistic regression analysis (p < 0.05).
Acknowledgements
We gratefully acknowledge Kirsten Pelz-Stelinski, Betsy Muellen (MSU), Robert Holdcraft, Vera Kyryczenko-Roth, Elizabeth Bender, Dean Polk and Cesar Rodriguez-Saona (RU) for collecting infested fruit and fly puparia. We thank Elizabeth Steere and Angel Hoyte for diligent maintenance of insect colonies.
Addendum to:
References
- Stelinski LL, Rodriguez-Saona C, Meyer WL. Recognition of foreign oviposition-marking pheromone in a multi-trophic context. Naturwissenschaften 2009; 96:585 - 592
- Ruther J, Meiners T, Steidle LM. Rich in phenomena-lacking in terms. A classification of kairomones. Chemoecology 2002; 12:161 - 167
- Vet LEM, Dicke M. Ecology of infochemical use by natural enemies in a tritrophic context. Annu Rev Entomol 1992; 37:141 - 172
- Nufio CR, Papaj DR. Host marking behavior in phytophagous insects and parasitoids. Entomol Exp Appl 2001; 99:273 - 293
- Roitberg BD, Prokopy RJ. Insects that mark host plants. Bioscience 1987; 37:400 - 406
- Hoffmeister TD, Roitberg BD. Counterespionage in an insect herbivore-parasitoid system. Naturwissenschaften 1997; 84:117 - 119
- Glas PCG, Vet LEM. Host-habitat location and host location by Diachasma alloeum Muesebeck (Hym.; Braconidae), a parasitoid of Rhagoletis pomonella Walsh (Dipt.; Tephritidae). Neth J Zool 1983; 33:41 - 54
- Stelinski LL, Pelz KS, Liburd OE. Field observations quantifying attraction of the parasitic wasp, Diachasma alloeum (Hymenoptera: Braconidae) to blueberry fruit infested by the blueberry maggot fly, Rhagoletis mendax (Diptera: Tephritidae). Fla Entomol 2004; 87:124 - 129
- Feder JL, Opp S, Wlazlo B, Reynolds K, Go W, Spisak S. Host fidelity is an effective pre-mating barrier between sympatric races of the apple maggot fly. Proc Natl Acad Sci USA 1994; 91:7990 - 7994
- Linn CE, Dambroski HR, Feder JL, Berlocher SH, Nojima S, Roelofs WL. Postzygotic isolating factor in sympatric speciation in Rhagoletis flies: reduced response of hybrids to parental host-fruit odours. Proc Natl Acad Sci USA 2004; 101:17753 - 17758
- Linn CE, Nojima S, Roelofs WL. Antagonistic effects of non-host fruit volatiles on discrimination of host fruit by Rhagoletis flies infesting apple (Malus pumila hawthorn, Crataegus spp.), and flowering dogwood (Cornus florida). Entomol Exp Appl 2005; 114:97 - 105
- Dambroski HR, Linn C, Berlocher S, Forbes AA, Roelofs W, Feder JL. The genetic basis for fruit odor discrimination in Rhagoletis flies and its significance for sympatric host shifts. Evolution 2005; 59:1953 - 1964
- Gandolfi M, Mattiacci L, Dorn S. Preimaginal learning determines adult response to chemical stimuli in a parasitic wasp. Proc R Soc Lond B 2003; 270:2623 - 2629
- Villagra CA, Pennacchio F, Niemeyer HM. The effect of larval and early adult experience on behavioral plasticity of the aphid parasitoid Aphidius ervi (Hymenoptera, Braconidae, Aphidiinae). Naturwissenschaften 2007; 94:903 - 910
- Nojima S, Linn CE Jr, Zhang A, Morris B, Roelofs WL. Identification of host fruit volatiles from hawthorn (Crataegus spp.) attractive to hawthorn-origin Rhagoletis pomonella flies. J Chem Ecol 2003; 29:319 - 334
- Roelofs WL. Hummel HE, Miller TA. Electroantennogram assays: rapid and convenient screening procedures for pheromones. Techniques in pheromone research 1984; New York Springer 131 - 159
- Prokopy RJ. Nordlund DA, Jones RL, Lewis WJ. Epideictic pheromones that influence spacing patterns of phytophagous insects. Semiochemicals: their role in pest control 1981; New York John Wiley & Sons 181 - 213
- Stelinski LL, Oakleaf R, Rodriguez-Saona C. Oviposition-deterring pheromone deposited on blueberry fruit by the parasitic wasp, Diachasma alloeum. Behaviour 2007; 144:429 - 445