Abstract
Chloroplasts are descended from a cyanobacterial endosymbiont and divide by binary fission. Reminiscent of the process in their bacterial ancestor, chloroplast division involves a part of cyanobacteria-derived division machineries in addition to those acquired during chloroplast evolution.1,2 In both bacterial and chloroplast division, formation of the FtsZ ring at the mid position is required for subsequent constriction and fission at the mid division site.1-4 As in bacteria, positioning of the FtsZ ring at the mid-chloroplast is mediated by the Min system.1,2 Recently, we identified the MCD1 protein, a plant-specific component of the Min system in Arabidopsis thaliana chloroplasts.5 Unlike other division components that have been acquired after endosymbiosis and function outside of the chloroplasts (i.e. in/on the outer envelope membrane),6-9 MCD1 functions inside the chloroplast. Since we already discussed about the function and significance of MCD1 as a division component of plant origin,5 here we focus on and discuss about the diversity and evolution of the Min system.
Components of the Min System in Escherichia coli and Bacillus subtilis
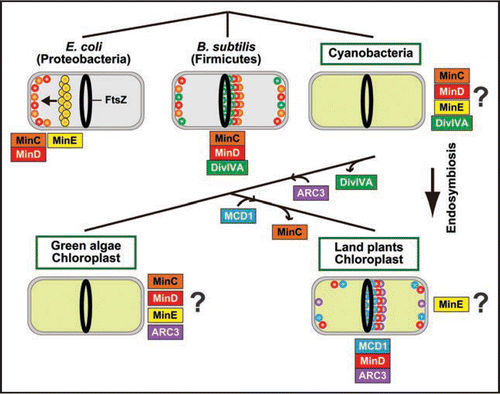
Bacterial cells usually divided binary division by constriction at the mid-cell position. The formation of a ring structure by polymerization of the tubulin-like protein FtsZ is the first known event at the division site, and it initiates the recruitment of the other proteins that comprise the bacterial division complex. The placement of the FtsZ ring at the mid-cell position is partly mediated by the Min system, which prevents FtsZ ring formation other than at the mid-cell position.Citation3,Citation4 A large number of genome projects have revealed both partial conservation and also certain differences in the composition of the Min system in diverse bacterial lineages.Citation3
The Min system has been studied extensively in both the proteobacterium (Gram-negative) Escherichia coli and firmicute (Gram-positive) Bacillus subtilis.Citation3,Citation4 In both organisms, MinC inhibits FtsZ ring formation, and MinD is a membrane-associated protein which recruits MinC to the membrane.Citation10–Citation13 The MinCD complex inhibits division other than at the mid-cell position, while allowing division at the mid-cell position.
Despite the conservative function of the MinCD complex, the topological specificity of the MinCD is regulated differently in E. coli and B. subtilis. In E. coli MinE sweeps the MinCD complex from one pole to the other so that MinCD undergoes subsequent pole-to-pole oscillation (). The time-averaged concentration of MinCD is maintained at a high level near the cell poles, and at a low level mid-cell. As a result, division is prevented at the cell poles and is only allowed at sites mid-cell.Citation14,Citation15 On the other hand, in B. subtilis, which does not have MinE, DivIVA recruits MinCD at the cell poles so that the FtsZ ring forms mid-cell. In B. subtilis, MinCD also localizes at the division site after FtsZ ring formation (),Citation16–Citation18 but the significance of the division-site localization is still unclear.
Components of the Min System in Cyanobacteria and Chloroplasts
Chloroplasts evolved from a cyanobacterial ancestor that was engulfed and enslaved by a eukaryotic host cell. Reminiscent of their bacterial ancestor, chloroplasts divide by binary division. Studies have shown that the chloroplast division involves FtsZ, MinD and MinE, genes which have been transferred from the engulfed cyanobacterium to the plant nuclear genome.Citation1,Citation2 Although the exact action of the Min system in cyanobacteria and chloroplasts has not been clarified, database searches and analyses of gene disruptants revealed that MinC, MinD, MinE and DivIVA-like protein are involved in cyanobacterial cell division ().Citation19,Citation20 Therefore, unlike the case in B. subtilis and E. coli, both MinE and DivIVA participate in cyanobacterial cell division.
Of the MinC, MinD, MinE and DivIVA-like proteins which are conserved in cyanobacteria, cyanobacteria-descended MinD and MinE are found in the genomes of green algae and land plants ().Citation1,Citation2 In addition, both have been shown to regulate the positioning of the FtsZ ring in the chloroplasts of A. thalianaCitation21,Citation22 and Chlamydomonas reinhardtii.Citation23 Putative MinC is evident in green algae (e.g., gi:145340725) and the moss Physcomitrella patens (gi:168012958) (),Citation2 although the function has yet to be characterized. These sequences are most closely related to those of cyanobacterial MinC, but missing in other land plant genomes, suggesting that MinC has been lost during the course of land plant evolution (). On the other hand, no DivIVA homolog has been found in the available plant and algal genome data, suggesting that DivIVA had been lost at an earlier stage of chloroplast evolution ().
Chloroplast-Specific Components of the Min System
In addition to MinD and MinE, studies on the A. thaliana chloroplast division mutants, arc3,Citation24,Citation25 and mcd1,Citation5 have identified additional proteins that regulate FtsZ ring positioning in concert with MinD and MinE. The ARC3 protein is composed of an incomplete FtsZ-like domain, a middle domain with no recognizable sequence motifs, and a domain with partial similarity to phosphatidylinositol-4-phosphate 5-kinase (PIP5K). The PIP5K-like region bears membrane occupation and recognition nexus (MORN) motifs, and is known to bind to membranes. ARC3 is reported to localize at the division site and poles of chloroplasts ().Citation24,Citation25 Similar to the A. thaliana minD mutant, arc3 mutation results in asymmetric chloroplast division and the formation of multiple FtsZ rings in single chloroplasts, indicative of a defect in FtsZ ring positioning.Citation24–Citation26 Similarly to bacterial MinC, ARC3 directly interacts with MinD, MinE and FtsZ, and overexpression of ARC3 inhibits FtsZ ring formation. Therefore, it is suggested that ARC3 fulfills the role of MinC, which is absent in land plants.Citation1,Citation2,Citation25
MCD1 spans the inner envelope membrane and bears a coiled-coil motif on the stromal side. Similar to the minD and arc3 mutants, mcd1 mutation results in asymmetric chloroplast division and the formation of multiple FtsZ rings in single chloroplasts.Citation5 MCD1 directly interact with MinD, recruiting MinD to the chloroplast division site and the punctate structures dispersed on the inner envelope ().Citation5 Like MCD1, DivIVA bears coiled-coil motifs and recruits MinCD, but the recruitment requires another component, MinJ, in B. subtilis,Citation27,Citation28 and there are no similarities between MCD1 and DivIVA in terms of amino acid sequence.
ARC3 is found in the genomes of both green algae and land plants, whereas MCD1 is specific to land plants, suggesting that ARC3 became integrated into the chloroplast Min system at a relatively earlier point of chloroplast evolution than MCD1 ().
Future Perspectives on Evolutionary and Comparative Analyses of the Min System
Although the experimental data are still limited in cyanobacteria and chloroplasts, the differences in composition of the Min system among bacteria, plant and algal chloroplasts suggest the existence of several different mechanisms to position the FtsZ ring. In addition, a comparison of the genome sequences along with the identification and characterization of ARC3,Citation24,Citation25 MinDCitation21,Citation29 and MCD1,Citation5 indicate that the chloroplast Min system has undergone stepwise reconstruction by the loss and acquisition of various components ().
For a better understanding the evolution of the chloroplast Min system, (1) information on action of the cyanobacterial Min system is indispensable. In A. thaliana chloroplasts, MinD localizes at the division site, as in B. subtilis ().Citation5 Together with the evident involvement of DivIVA in cyanobacteria as in B. subtilis, this suggests that cyanobacterial MinCD localizes at the division site and cell poles, as do MinCD in B. subtilis. However, MinE is involved in cyanobacterial and chloroplast division, unlike the case in B. subtilis. (2) The localization of MinE in cyanobacteria and chloroplasts will provide critical insights into an understanding of the divergence of the Min system. During the course of chloroplast evolution, ARC3 was acquired and the MinC-like protein was lost (). It is also notable that certain bacterial and archaeal species have MinD, but not MinC.Citation30 (3) Functional experiments on the MinC-like protein, and comparison between action of MinC and ARC3, will reveal how Min system works without MinC, which is a division inhibitor therefore an effector of the Min system in bacteria. As above, there are many points that remain to be addressed. Further studies should provide important insights into the commonalities and differences in the bacterial and chloroplast Min systems.
Addendum to:
References
- Maple J, Moller SG. Plastid division coordination across a double-membraned structure. FEBS Lett 2007; 581:2162 - 2167
- Yang Y, Glynn JM, Olson BJ, Schmitz AJ, Osteryoung KW. Plastid division: across time and space. Curr Opin Plant Biol 2008; 11:577 - 584
- Rothfield L, Taghbalout A, Shih YL. Spatial control of bacterial division-site placement. Nat Rev Microbiol 2005; 3:959 - 968
- Harry E, Monahan L, Thompson L. Bacterial cell division: the mechanism and its precison. Int Rev Cytol 2006; 253:27 - 94
- Nakanishi H, Suzuki K, Kabeya Y, Miyagishima SY. Plant-specific protein MCD1 determines the site of chloroplast division in concert with bacteria-derived MinD. Curr Biol 2009; 19:151 - 156
- Miyagishima SY, Nishida K, Mori T, Matsuzaki M, Higashiyama T, Kuroiwa H, et al. A plant-specific dynamin-related protein forms a ring at the chloroplast division site. Plant Cell 2003; 15:655 - 665
- Gao H, Kadirjan-Kalbach D, Froehlich JE, Osteryoung KW. ARC5, a cytosolic dynamin-like protein from plants, is part of the chloroplast division machinery. Proc Natl Acad Sci USA 2003; 100:4328 - 4333
- Miyagishima SY, Froehlich JE, Osteryoung KW. PDV1 and PDV2 mediate recruitment of the dynamin-related protein ARC5 to the plastid division site. Plant Cell 2006; 18:2517 - 2530
- Glynn JM, Froehlich JE, Osteryoung KW. Arabidopsis ARC6 coordinates the division machineries of the inner and outer chloroplast membranes through interaction with PDV2 in the intermembrane space. Plant Cell 2008; 20:2460 - 2470
- Marston AL, Thomaides HB, Edwards DH, Sharpe ME, Errington J. Polar localization of the MinD protein of Bacillus subtilis and its role in selection of the mid-cell division site. Genes Dev 1998; 12:3419 - 3430
- Marston AL, Errington J. Selection of the midcell division site in Bacillus subtilis through MinD-dependent polar localization and activation of MinC. Mol Microbiol 1999; 33:84 - 96
- Hu Z, Mukherjee A, Pichoff S, Lutkenhaus J. The MinC component of the division site selection system in Escherichia coli interacts with FtsZ to prevent polymerization. Proc Natl Acad Sci USA 1999; 96:14819 - 14824
- Hu Z, Gogol EP, Lutkenhaus J. Dynamic assembly of MinD on phospholipid vesicles regulated by ATP and MinE. Proc Natl Acad Sci USA 2002; 99:6761 - 6766
- Hu Z, Lutkenhaus J. Topological regulation of cell division in Escherichia coli involves rapid pole to pole oscillation of the division inhibitor MinC under the control of MinD and MinE. Mol Microbiol 1999; 34:82 - 90
- Raskin DM, de Boer PA. MinDE-dependent pole-to-pole oscillation of division inhibitor MinC in Escherichia coli. J Bacteriol 1999; 181:6419 - 6424
- Cha JH, Stewart GC. The divIVA minicell locus of Bacillus subtilis. J Bacteriol 1997; 179:1671 - 1683
- Edwards DH, Errington J. The Bacillus subtilis DivIVA protein targets to the division septum and controls the site specificity of cell division. Mol Microbiol 1997; 24:905 - 915
- Gregory JA, Becker EC, Pogliano K. Bacillus subtilis MinC destabilizes FtsZ-rings at new cell poles and contributes to the timing of cell division. Genes Dev 2008; 22:3475 - 3488
- Mazouni K, Domain F, Cassier-Chauvat C, Chauvat F. Molecular analysis of the key cytokinetic components of cyanobacteria: FtsZ, ZipN and MinCDE. Mol Microbiol 2004; 52:1145 - 1158
- Miyagishima SY, Wolk CP, Osteryoung KW. Identification of cyanobacterial cell division genes by comparative and mutational analyses. Mol Microbiol 2005; 56:126 - 143
- Colletti KS, Tattersall EA, Pyke KA, Froelich JE, Stokes KD, Osteryoung KW. A homologue of the bacterial cell division site-determining factor MinD mediates placement of the chloroplast division apparatus. Curr Biol 2000; 10:507 - 516
- Itoh R, Fujiwara M, Nagata N, Yoshida S. A chloroplast protein homologous to the eubacterial topological specificity factor minE plays a role in chloroplast division. Plant Physiol 2001; 127:1644 - 1655
- Adams S, Maple J, Moller SG. Functional conservation of the MIN plastid division homologues of Chlamydomonas reinhardtii. Planta 2008; 227:1199 - 1211
- Shimada H, Koizumi M, Kuroki K, Mochizuki M, Fujimoto H, Ohta H, et al. ARC3, a chloroplast division factor, is a chimera of prokaryotic FtsZ and part of eukaryotic phosphatidylinositol-4-phosphate 5-kinase. Plant Cell Physiol 2004; 45:960 - 967
- Maple J, Vojta L, Soll J, Moller SG. ARC3 is a stromal Z-ring accessory protein essential for plastid division. EMBO Rep 2007; 8:293 - 299
- Glynn JM, Miyagishima SY, Yoder DW, Osteryoung KW, Vitha S. Chloroplast division. Traffic 2007; 8:451 - 461
- Patrick JE, Kearns DB. MinJ (YvjD) is a topological determinant of cell division in Bacillus subtilis. Mol Microbiol 2008; 70:1166 - 1179
- Bramkamp M, Emmins R, Weston L, Donovan C, Daniel RA, Errington J. A novel component of the division-site selection system of Bacillus subtilis and a new mode of action for the division inhibitor MinCD. Mol Microbiol 2008; 70:1556 - 1569
- Fujiwara MT, Nakamura A, Itoh R, Shimada Y, Yoshida S, Moller SG. Chloroplast division site placement requires dimerization of the ARC11/AtMinD1 protein in Arabidopsis. J Cell Sci 2004; 117:2399 - 2410
- Rothfield L, Justice S, García-Lara J. Bacterial cell division. Annu Rev Genet 1999; 33:423 - 448