Abstract
Systemic homeostasis requires coordinated metabolic regulation among multiple tissues/organs via inter-organ communication. We have reported that neuronal signaling plays important roles in this inter-organ metabolic communication. First, we found that liver-selective extracellular signal-regulated kinase (ERK) activation induces insulin hypersecretion and pancreatic β cell proliferation. Denervation experiments revealed that these inter-organ (liver-to-pancreas) effects are mediated by a neural relay consisting of splanchnic afferents (from the liver) and vagal efferents (to the pancreas). The central nervous system also participates in this inter-organ communication. This neural relay system originating in the liver is physiologically involved in the anti-diabetes mechanism whereby, during obesity development, insulin hypersecretion and pancreatic β cell hyperplasia occur in response to insulin resistance. This indicates the pathophysiological importance of this system in diabetes prevention and hyperinsulinemia development. Furthermore, when applied to mouse models of insulin-deficient diabetes, both type 1 and type 2, hepatic activation of ERK signaling increased pancreatic β cell mass and normalized blood glucose. Thus, this inter-organ system may serve as a valuable therapeutic target for diabetes by regenerating pancreatic β cells. The concept that manipulation of an endogenous mechanism can regenerate a damaged tissue in vivo may open a new paradigm for regenerative trreatments for degenerative disorders.
In multi-organ organisms, including human beings, metabolism in different tissues and organs does not go on independently, but rather in a coordinated and regulated manner throughout the body. This coordinated metabolic regulation requires inter-organ metabolic communication and is apparently essential for maintaining systemic homeostasis, particularly glucose and energy metabolism.Citation1 Therefore, communication among organs/tissues is extremely important and perturbation of this control system may lead to the development of metabolic disorders. During this decade, the versatility of adipose tissue as an endocrine organ and as a contributor to disease development has been established. In this context, humoral factors, including adipokines, are known to play important roles in this communication. However, a number of recent studies have shown that tissue-specific knockout and transgenic mice exhibit unexpected metabolic phenotypes in other tissues,Citation2–Citation5 suggesting the presence of as yet unknown metabolic communication systems.
Recently, several reports, including ours, have indicated that neuronal signaling, consisting of both afferent and efferent autonomic nerves, plays important roles in inter-organ metabolic communication and systemic homeostasis.Citation6 For instance, neuronal signals from visceral adipose tissue modulate food intake,Citation7 while those from the liver regulate energy expenditure.Citation8 In addition to these anti-obesity neuronal mechanisms, we have further identified a neuronal relay, originating in the liver, which enhances pancreatic β cell proliferation and thus functions as an endogenous anti-diabetes mechanism.
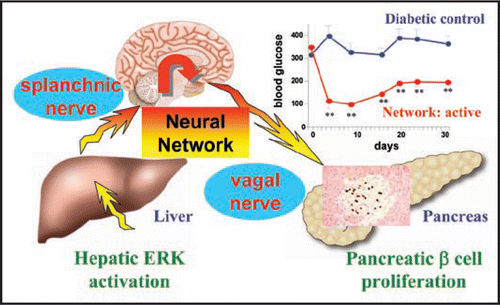
Obesity induces insulin hypersecretion and pancreatic β cell hyperplasia in response to insulin resistance. These compensatory responses of pancreatic β cells protect individuals from the development of diabetes but induce hyperinsulinemia which is involved in the pathological phenotypes of the metabolic syndrome. To elucidate the mechanisms underlying the compensatory pancreatic β cell responses, we activated proteins, which are reportedly activated in the livers of obesity models, in the livers of lean mice. Among them, hepatic signaling of extracellular signal-regulated kinase (ERK), phosphorylation of which is reportedly enhanced in the liver of a murine obesity model,Citation9,Citation10 was shown to play an important role in compensatory pancreatic β cell responses. To activate ERK in the liver, constitutively active mutant of mitogen-activated protein kinase/ERK kinase (MEK-1) was expressed in the liver using an adenoviral gene transduction system.Citation11 Intriguingly, liver-selective ERK activation induced insulin hypersecretion and pancreatic β cell proliferation. These pancreatic effects of hepatic ERK activation were inhibited by either splanchnic afferent blockade, pancreatic vagus dissection or midbrain transection. These results indicate that a neuronal relay system, consisting of the afferent splanchnic nerve, the central nervous system and the efferent vagus, mediates inter-organ (liver-to-pancreas) communication. In addition, blockade of this neuronal relay at each of several steps in murine obesity models inhibited pancreatic islet expansion during obesity development, showing the physiological role of this inter-organ mechanism in compensatory pancreatic β cell responses to obesity-induced insulin resistance. Furthermore, when applied to mouse models of insulin-deficient diabetes, hepatic activation of ERK signaling induced pancreatic β cell regeneration and thereby improved diabetes.
Our Study Highlights Several Novel and Important Points
First, pancreatic β cell mass was shown to be regulated by a neural relay originating in the liver. The liver is likely to sense metabolic conditions requiring insulin hypersecretion and to send signals via the neuronal information highway. This novel inter-organ mechanism may play very important roles in glucose homeostasis by regulating insulin secretion.
Second, involvement of afferent signals underscores the importance of the central nervous system in maintaining metabolic homeostasis. Afferent signals are received at the brainstem including the medulla and transferred to the secondary neurons which pass through the midbrain. Since midbrain transection blocks the pancreatic effects induced by hepatic ERK activation, the metabolic information originating in the liver is likely to be conveyed from the brainstem possibly to the diencephalon, including the hypothalamus and processed in the “metabolic center” in the diencephalon, resulting in transmission of signals inducing proliferation of pancreatic β cells via efferent nerves (). Thus, the brain may obtain various forms of metabolic information from peripheral organs/tissues, on a constant basis, and then transmit regulatory signals to peripheral tissues/organs throughout the body to induce appropriate systemic responses.
Third, this inter-organ machinery was shown to physiologically elicit compensatory islet responses to insulin resistance associated with obesity. These responses occur prior to hyperglycemia development, and thereby prevent diabetes during obesity development. However, this anti-diabetes mechanism induces hyperinsulinemia and in turn, ironically, contributes to development of the metabolic syndrome. Thus, this neural relay system is pathophysiologically involved in type 2 diabetes and the metabolic syndrome.
Finally, we would like to emphasize the implications and significance of therapeutic application to diabetes. Type 1 diabetes is characterized by severe pancreatic β cell loss. Decreases in pancreatic β cell mass are also reported in patients with type 2 diabetes.Citation12 In these patients, one potential underlying mechanism is β cell apoptosis induced by endoplasmic reticulum (ER) stress.Citation13–Citation15 In this study, we used two mouse models of insulin-deficient diabetes, induced by severe pharmacological pancreatic β cell loss (type 1 diabetes model) and by ER stress-induced β cell apoptosis (type 2 diabetes model). In both murine models, liver-selective activation of ERK signaling resulted in an increase in β cell mass and normalization of serum glucose levels. Thus, this inter-organ system may serve as a valuable therapeutic target for diabetes, both type 1 and type 2, by regenerating pancreatic β cell mass. For regenerative medicine, many researchers are endeavoring to develop strategies whereby multi-potent cells, such as embryonic stem (ES)16 and induced pluripotent stem (iPS)Citation17 cells, differentiate into an intended organ in vitro. In contrast, our study showed that stimulation of a neural machinery increased pancreatic β cells which had previously diminished. Thus, this is an example whereby manipulation of endogenous neural machinery can lead to regeneration of a damaged tissue in vivo. This concept may open a new paradigm for regenerative medicine not only for diabetes but also many other degenerative disorders.
Figures and Tables
Figure 1 Schematic model of the neural relay originating in the liver (for details see text). Hepatic ERK activation associated with obesity results in pancreatic β cell proliferation, via the neuronal system consisting of afferent and efferent nerves and the central nervous system. When applied to mouse models of insulin-deficient diabetes, activation of this neural relay normalized blood glucose. This inter-organ system may serve as a valuable therapeutic target for diabetes by regenerating pancreatic β cell mass.
Addendum to:
References
- Katagiri H, Yamada T, Oka Y. Adiposity and cardiovascular disorders: disturbance of the regulatory system consisting of humoral and neuronal signals. Circ Res 2007; 101:27 - 39
- Abel ED, Peroni O, Kim JK, Kim YB, Boss O, Hadro E, et al. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature 2001; 409:729 - 733
- Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, et al. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell 2000; 6:87 - 97
- Brüning JC, Michael MD, Winnay JN, Hayashi T, Hörsch D, Accili D, et al. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell 1998; 2:559 - 569
- Blüher M, Michael MD, Peroni OD, Ueki K, Carter N, Kahn BB, et al. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev Cell 2002; 3:25 - 38
- Yamada T, Oka Y, Katagiri H. Inter-organ metabolic communication involved in energy homeostasis: potential therapeutic targets for obesity and metabolic syndrome. Pharmacol Ther 2008; 117:188 - 198
- Yamada T, Katagiri H, Ishigaki Y, Ogihara T, Imai J, Uno K, et al. Signals from intra-abdominal fat modulate insulin and leptin sensitivity through different mechanisms: neuronal involvement in food-intake regulation. Cell Metab 2006; 3:223 - 229
- Uno K, Katagiri H, Yamada T, Ishigaki Y, Ogihara T, Imai J, et al. Neuronal pathway from the liver modulates energy expenditure and systemic insulin sensitivity. Science 2006; 312:1656 - 1659
- Yang S, Lin HZ, Hwang J, Chacko VP, Diehl AM. Hepatic hyperplasia in noncirrhotic fatty livers: is obesity-related hepatic steatosis a premalignant condition?. Cancer Res 2001; 61:5016 - 5023
- GGum RJ, Gaede LL, Heindel MA, Waring JF, Trevillyan JM, Zinker BA, et al. Antisense protein tyrosine phosphatase 1B reverses activation of p38 mitogen-activated protein kinase in liver of ob/ob mice. Mol Endocrinol 2003; 17:1131 - 1143
- Fujishiro M, Gotoh Y, Katagiri H, Sakoda H, Ogihara T, Anai M, et al. Three mitogen-activated protein kinases inhibit insulin signaling by different mechanisms in 3T3-L1 adipocytes. Mol Endocrinol 2003; 17:487 - 497
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003; 52:102 - 110
- Harding HP, Ron D. Endoplasmic reticulum stress and the development of diabetes: a review. Diabetes 2002; 51:455 - 461
- Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest 2002; 110:1389 - 1398
- Ishihara H, Takeda S, Tamura A, Takahashi R, Yamaguchi S, Takei D, et al. Disruption of the WFS1 gene in mice causes progressive beta-cell loss and impaired stimulus-secretion coupling in insulin secretion. Hum Mol Genet 2004; 13:1159 - 1170
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science 1998; 282:1145 - 1147
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007; 131:861 - 872