Abstract
The C-terminal Eps15 homology domain-containing protein, EHD1, is an important regulator of receptor recycling back to the plasma membrane. In addition to its vesicular localization, EHD1 also localizes to a unique array of tubular membrane structures that emanate from the endocytic recycling compartment. While these structures have been described over 7 years ago, addressing their lipid composition and physiological function has been challenging. Moreover, it was not known whether EHD1 itself induces tubule formation, or whether it localizes to pre-existing tubular membrane structures. We have demonstrated that in vivo, EHD1 localizes to pre-existing tubular membranes that contain both phosphatidylinositol-4-phosphate and phosphatidylinositol-(4,5)-bisphosphate. Moreover, we have determined that ‘non-tubular’ EHD1 mutants with a single residue substitution do not efficiently facilitate receptor recycling. Our data suggest that EHD1-associated tubules are required for efficient recycling and we propose models that describe the potential mechanisms by which EHD1 functions.
Endocytic recycling is the major route by which internalized proteins and lipids can be returned back to the cell surface. It is thus essential for maintenance of membrane homeostasis and requires exquisite coordination by a multitude of regulatory proteins. The Rab family of GTP-binding proteins and their effectors play crucial roles in regulation of endocytic recycling events. Rab4 regulates the ‘fast recycling’ of cargo directly from early endosomes as well as trafficking of cargo from early endosomes to the endocytic recycling compartment (ERC), while Rab11 regulates exit of slow recycling cargo from ERC back to the plasma membrane (reviewed in ref. Citation1). Endocytic activity is also regulated by the C-terminal Eps15 Homology Domain (EHD) family of proteins. In mammalian cells, four paralogs of EHD proteins (EHD1-4) are expressed and function at distinct steps in endocytic trafficking.Citation2,Citation3 EHD1, the best characterized mammalian EHD protein has a primary role in regulating receptor recycling from the ERC to the plasma membraneCitation4–Citation9 and coordinates its activity with effectors of Rab4 and Rab11.Citation10,Citation11
Although EHD proteins contain an N-terminal G domain similar to the GTP-binding Ras-family of proteins, they bind and hydrolyze ATP, and this is essential for their oligomerization and localization to tubular and vesicular membranes.Citation5,Citation11–Citation13 EHD proteins also contain a central helical domain that binds to phosphoinositides.Citation12 The C-terminal region of the protein is characterized by an EH-domain, comprised of a pair of EF-Hand helix-loop-helix motifs linked by a short antiparallel β-sheet.Citation12,Citation14 One of the hallmarks of EHD1 is its distribution to cytosolic face of long tubular membranes and vesicles that emanate from the ERC. The localization to tubular membranes requires the presence of the EH domain,Citation5 which is capable of interacting with phosphoinositides.Citation15 Indeed, our recent NMR solution structure of the EHD1 EH-domain demonstrates that lysine 483 (within the EH domain) is important for phosphoinositide association.Citation15 Consequently, a charge-reversal substitution of this residue to glutamate (K483E) prevents association of EHD1 with tubular membranes, similar to truncation of the entire 100 amino acid EH-domain.Citation15 However, until recently, it had not been established whether EHD1 tubule association was required for its function in receptor recycling. To address this, we took advantage of our previous studies demonstrating that depletion of EHD1 resulted in the accumulation of internalized receptors at the ERC, causing a delay in their recycling back to the plasma membrane.Citation10 To assess the role of EHD1-tubules in receptor recycling, we treated cells with EHD1-SiRNA and ‘rescued’ its impaired recycling by reintroducing SiRNA-resistant wild-type EHD1. However, when the non-tubule-associated EHD1 K483E mutant was introduced into the depleted cells, very little rescue of receptor recycling was observed. These experiments demonstrate that EHD1-containing tubules are necessary for efficient receptor recycling to the plasma membrane.Citation9
The EH domain of EHD1 can bind various phosphoinositides in vitro.Citation15 To analyze the phosphoinositide composition of EHD1-containing tubules in vivo, we developed a novel strategy to manipulate phosphoinositide levels in cells. We found that phosphatidylinositol-4-phosphate (PtdIns4P) is a critical component of EHD1-tubules, as depletion of this lipid either by overexpression of Sac1, a PtdIns4P-phosphatase, or by overexpression of the PtdIns4P kinase, PIP5KIγ {which converts PtdIns4P to phosphatidylinositiol-(4,5)-bisphosphate [PtdIns(4,5)P2]}, disrupts localization of EHD1 to tubular membranes.Citation9 We also observed that PtdIns4P (detected using specific antibodies) co-localized with endogenous EHD1-containing tubules. Additionally, we transfected protein domains that bind specific phosphoinositides and analyzed their co-localization with EHD1-containing tubular membranes. Partial co-localization of EHD1-tubules was observed with the PH domain of OSBP (a marker for PtdIns4P) and the PLCδ1 PH-domain [a marker of PtdIns(4,5)P2]. These data support our contention that EHD1-containing tubules are comprised of PtdIns4P as well as levels of PtdIns(4,5)P2.
Since the initial report that EHD1 localizes to membrane tubules,Citation5 a key unresolved issue has been whether EHD1 induces generation of these structures, or decorates existing membranes. A recent study argued for the former possibility, showing that EHD proteins are intrinsically capable of tubulating membranes in vitro.Citation12 By using GFP fused to the double palmitoylated and farnesylated carboxyl terminal tail of H-Ras (GFP-H-Ras), which associates with tubular membranes of the clathrin-independent endocytic pathway,Citation16 and Rab8a, which localizes to EHD1-containing tubular membranes,Citation17 we analyzed the localization of these two proteins to tubules in cells depleted of EHD1. As their localization remained unchanged and they continued to associate with tubular membranes even in the absence of EHD1, we concluded that EHD1 associates with pre-existing tubular membranes in vivo.Citation9
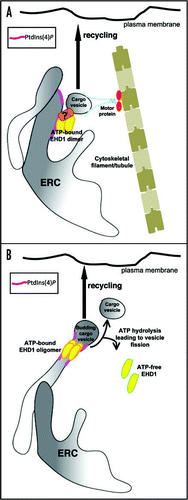
In conclusion although, EHD proteins are capable of generating tubules in vitro, EHD1 associates, at least in part, with pre-existing PtdIns4P-containing tubular membranes in vivo.Citation9 We also provide the first evidence of a role for the EHD1-containing tubules for maintaining efficient recycling to the plasma membrane. How might EHD1 localization to these structures facilitate efficient receptor recycling? One possibility is that EHD1 might associate with the cargo vesicles arriving from the early endosome on the tubular ERC membranes and link them to motor proteins on cytoskeletal tracks (). The motor proteins would then drive these cargo vesicles for delivery back to the plasma membrane. A second, but not mutually exclusive possibility, relies on the potential ability of EHD proteins to mediate membrane fission in response to ATP hydrolysis. In this scenario, ATP-bound EHD1 oligomerizes and constricts the neck of a cargo vesicle budding from the ERC; upon ATP hydrolysis membrane fission occurs, releasing the vesicle and allowing its transport back to the plasma membrane (). Validation of these models remains a high priority for future studies.
Figures and Tables
Figure 1 Potential models depicting mechanisms of EHD1-mediated recycling from the ERC. (A) EHD1 dimers in their ATP-bound state are recruited to the PtdIns(4)P-enriched tubular membranes of the ERC. EHD1 can then either directly or indirectly associate with cargo vesicles (via an unknown protein(s) such as a Rab effector) coming from the early endosome and provide a platform for association of motor proteins that might drive recycling vesicles back to the plasma membrane. (B) A second proposed mechanism for EHD1-mediated recycling is that ATP-bound EHD1 associates with tubular PtdIns(4)P-enriched membranes. Once on these structures, EHD1 utilizes its intrinsic ATPase activity and acts as a ‘pinchase’ to promote membrane fission of the vesicles budding from the ERC tubules. These pinched-off cargo vesicles can then be carried back to the plasma membrane by the motor proteins as described above.
Acknowledgements
We gratefully acknowledge the support of NIH grants GM074876 and P20 RR018759 (SC), GM072631 (PLS), and the American Heart Association (MS).
Addendum to:
References
- Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol 2004; 5:121 - 132
- Grant BD, Caplan S. Mechanisms of EHD/RME-1 protein function in endocytic transport. Traffic 2008; 9:2043 - 2052
- Naslavsky N, Caplan S. C-terminal EH-domain-containing proteins: consensus for a role in endocytic trafficking, EH?. J Cell Sci 2005; 118:4093 - 4101
- Lin SX, Grant B, Hirsh D, Maxfield FR. Rme-1 regulates the distribution and function of the endocytic recycling compartment in mammalian cells. Nat Cell Biol 2001; 3:567 - 572
- Caplan S, Naslavsky N, Hartnell LM, Lodge R, Polishchuk RS, Donaldson JG, et al. A tubular EHD1-containing compartment involved in the recycling of major histocompatibility complex class I molecules to the plasma membrane. EMBO J 2002; 21:2557 - 2567
- Guilherme A, Soriano NA, Furcinitti PS, Czech MP. Role of EHD1 and EHBP1 in perinuclear sorting and insulin-regulated GLUT4 recycling in 3T3-L1 adipocytes. J Biol Chem 2004; 279:40062 - 40075
- Picciano JA, Ameen N, Grant BD, Bradbury NA. Rme-1 regulates the recycling of the cystic fibrosis transmembrane conductance regulator. Am J Physiol Cell Physiol 2003; 285:1009 - 1018
- Park M, Penick EC, Edwards JG, Kauer JA, Ehlers MD. Recycling endosomes supply AMPA receptors for LTP. Science 2004; 305:1972 - 1975
- Jović M, Kieken F, Naslavsky N, Sorgen PL, Caplan S. EHD1-associated tubules contain phosphatidylinositol-4-phosphate and phosphatidylinositol-(4,5)-bisphosphate and are required for efficient recycling. Mol Biol Cell 2009; 20:2731 - 2743
- Naslavsky N, Boehm M, Backlund PS Jr, Caplan S. Rabenosyn-5 and EHD1 interact and sequentially regulate protein recycling to the plasma membrane. Mol Biol Cell 2004; 15:2410 - 2422
- Naslavsky N, Rahajeng J, Sharma M, Jovic M, Caplan S. Interactions between EHD Proteins and Rab11-FIP2: A Role for EHD3 in Early Endosomal Transport. Mol Biol Cell 2006; 17:163 - 177
- Daumke O, Lundmark R, Vallis Y, Martens S, Butler PJ, McMahon HT. Architectural and mechanistic insights into an EHD ATPase involved in membrane remodelling. Nature 2007; 449:923 - 927
- Lee DW, Zhao X, Scarselletta S, Schweinsberg PJ, Eisenberg E, Grant BD, et al. ATP Binding regulates oligomerization and endosome association of RME-1 family proteins. J Biol Chem 2005; 280:280 - 290
- Kieken F, Jovic M, Naslavsky N, Caplan S, Sorgen PL. EH domain of EHD1. J Biomol NMR 2007; 39:323 - 329
- Naslavsky N, Rahajeng J, Chenavas S, Sorgen PL, Caplan S. EHD1 and Eps15 interact with phosphatidylinositols via their Eps15 homology domains. J Biol Chem 2007; 282:16612 - 16622
- Porat-Shliom N, Kloog Y, Donaldson JG. A unique platform for H-Ras signaling involving clathrin-independent endocytosis. Mol Biol Cell 2008; 19:765 - 775
- Roland JT, Kenworthy AK, Peranen J, Caplan S, Goldenring JR. Myosin Vb interacts with Rab8a on a tubular network containing EHD1 and EHD3. Mol Biol Cell 2007; 18:2828 - 2837