Abstract
The two fundamental lineages of photoreceptor cells, microvillar and ciliary, were long thought to be a prerogative of invertebrate and vertebrate organisms, respectively. However evidence of their ancient origin, preceding the divergence of these two branches of metazoa, suggests instead that they should be ubiquitously distributed. Melanopsin-expressing ‘circadian’ light receptors may represent the remnants of the microvillar photoreceptors amongst vertebrates, but they lack the characteristic architecture of this lineage, and much remains to be clarified about their signaling mechanisms. Hesse and Joseph cells of the neural tube of amphioxus (Branchiostoma fl.) – the most basal chordate extant - turn out to be depolarizing primary microvillar photoreceptors, that generate a melanopsin-initiated, PLC-dependent response to light, mobilizing internal Ca and increasing a membrane conductance selective to Na and Ca ions. As such, they represent a canonical instance of invertebrate-like visual cells in the chordate phylum.
The structural diversity of visual organs in animals is staggering, but at the level of photoreceptor cells things become simpler, and one encounters just a two-way partition defined by the structure of the light-sensing organelle: this is either comprised of microvilli, short infoldings of the apical membrane packed with actin filaments, or else it arises from a modified cilium, with its characteristic radial arrangement of microtubules.Citation1 These two cell types also differ radically in the biochemical scheme they have developed to couple photon absorption to the changes in ionic conductances that convert it into an electrical signal: invariably, microvillar receptors utilize phospholipase C (PLC) and phosophoinositide-based lipid signaling,Citation2 whereas ciliary receptors mobilize cyclic nucleotides.Citation3,Citation4
Traditionally, it was thought that this distinction was tightly associated with taxonomy—vertebrate retinas being comprised of ciliary receptors, and microvillar photoreceptors being strictly segregated to the eyes of invertebrates.Citation1 This vertebrate-invertebrate dichotomy is a puzzle in the light of the discovery, among others, of putative photoreceptors of the microvillar type in plathyelminthesCitation5 and both ciliary and microvillar in cnidariaCitation6 (see ). The presence of both classes of visual cells even in pre-bilateria implies that their origin must date back to a time preceding the separation of protostomia and deuterostomia. Therefore, descendants of both lineages of visual cells ought to be represented across the two branches. Indeed, bona fide ciliary photoreceptors have been studied in a few marine mollusksCitation7,Citation8 and likely candidates have been described in a growing number of other invertebrates,Citation9,Citation10 although their alleged light sensitivity is yet to be corroborated physiologically. By contrast, their counterparts, microvillar receptors, were never found in vertebrates.
The situation changed with the discovery that the retina of mammals contains previously unsuspected light-sensitive cells,Citation11 other than the familiar rods and cones. These comprise a small subclass of retinal ganglion cells (RGCs), the last relay station in the eye that sends information to the brain, and were dubbed intrinsically photosensitive retinal ganglion cells (ipRGCs); ipRGCs mediate a host of non-visual light-dependent functions, such as photo-entraining circadian rhythmsCitation12 and controlling the pupillary reflex.Citation13 The light-absorbing molecule that mediates the photoresponse of ipRGCs, melanopsin, turned out to be significantly more similar to the photopigments of invertebrate microvillar photoreceptors than those of vertebrates.Citation14 Other molecular and developmental markers, such as expression of transcription factors BarH and Brn3, also suggest a kinship between ipRGCs and their invertebrate cousins.Citation15 The detailed chemical cascade that couples light stimulation to the electrical response proved difficult to investigate in native cells, owing to their extreme scarcity, although clues are emerging that PLC is implicated.Citation16,Citation17 However, the characteristic microvillar morphology is conspicuously absent in ipRGCs, leaving considerable uncertainty about the presence and evolutionary history of microvillar receptors within the vertebrate phylum.
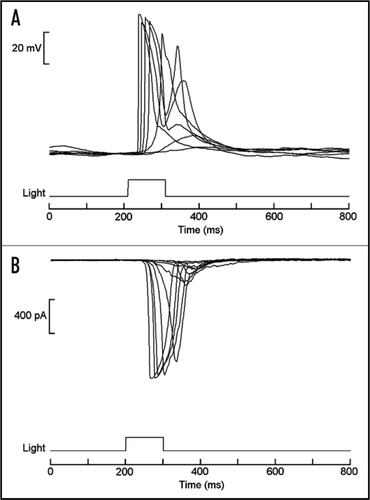
One possible strategy to search for the missing descendants of microvillar photoreceptors amongst deuterostomia is to focus on some early chordate, in which characteristic traits of the light-sensor may have been unambiguously retained. Amphioxus () is a primitive marine organism of great importance in evolutionary studies. Its genome has been recently sequencedCitation18 and molecular phylogeny has established that it is the most basal of all living chordates;Citation19 most important, it seemingly remains close to its ancestral condition, having changed little in the last half a million years. As such, it provides an unusually favorable window to examine biological mechanisms that may have been present in the ancestors of vertebrates. However, no functional study had been conducted on this species. Melanopsin was recently detected in the neural tube of amphioxus,Citation20 its expression pattern coinciding with two previously described clusters of microvilli-bearing cells: Joseph cells and organs of Hesse.Citation21–Citation23 This observation set the stage for a recent study of single-cell physiology.Citation24 Morphologically intact cells of both types were isolated ( and C), and electrophysiological recording was used to determine that they respond to light in the absence of all synaptic input (), thus establishing that they are indeed primary photoreceptors. The action spectrum matches that of melanopsin, peaking in the vicinity of 470 nm. Light mobilizes calcium from intracellular stores, as revealed by digital fluorescence imaging, and increases the permeability of the membrane to sodium and calcium ions, just like in microvillar receptors of insects and mollusks.Citation25
Additionally, key signaling molecules of the biochemical cascade that operates in invertebrate photoreceptors have been detected by western blot analysis, and the light response was shown to be susceptible to pharmacological agents that antagonize the PLC signaling pathway.Citation26 The results firmly establish the presence of bona fide microvillar receptors among chordates, and confirm that melanopsin utilizes the same biochemical signaling mechanisms found in invertebrates; as such, amphioxus Hesse and Joseph cells can be viewed as bridging the gap between the melanopsin-expressing circadian receptors of mammals and their microvillar cousins in invertebrate eyes.
The scenario may be ripe to re-visit some basic questions related to the evolutionary origin of photoreceptors cells. Vision had previously been thought to have arisen independently several dozen times throughout animal evolutionCitation27—a reasonable proposition in view of the strong evolutionary pressure and the undeniable competitive advantage conferred by the ability to exploit the abundantly available electromagnetic radiation for information-gathering purposes. However, this conjecture became difficult to sustain with the realization that the early signaling elements of the light-transduction cascade in distant species are orthologous,Citation15 even across the microvillar-ciliary boundary; this applies to the photopigment, the G-protein, and arrestin. In fact, examination of the transduction mechanisms in photoreceptors of bivalve marine mollusksCitation28 and jellyfishCitation29 indicates that the variety of light-signaling schemes amongst animals is actually richer than previously suspected, and yet in all cases a similar general blueprint is followed. The alternative, at the opposite end of the spectrum, is therefore a monophyletic origin, which naturally raises the question of which cell type may have been the ‘original’ photoreceptor. Spatial vision necessarily calls for directional sensitivity, and in its most primitive form it would entail a light sensor shielded on one side by a screen, as first envisioned by Darwin.Citation30 Because in all known primordial pigmented ocelli microvillar photoreceptors are implicated, it has been argued that the ancestral proto-eye may have consisted of a single microvillar photoreceptor associated with a pigmented cell,Citation31,Citation32 i.e., exactly like the organ of Hesse of present-day amphioxus.
Figures and Tables
Figure 1 Simplified phylogenetic tree. Ciliary photoreceptors are typical of vertebrata (in the chordata phylum), whereas microvillar photoreceptors have been extensively characterized both morphologically and physiologically in arthropoda and mollusca. However, putative photoreceptors of both types have subsequently been identified also in pre-bilateria, such as box jellyfish (cnidaria).
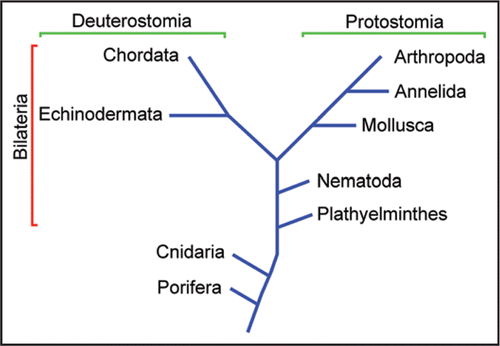
Figure 2 The amphioxus (Branchiostoma floridae). (A) Intact specimen. Calibration bar: 5 mm (B) Joseph cell enzymatically dissociated from the neural tube (the shadow is a recording patch microelectrode). (C) Isolated organ of Hesse, comprised of a pigmented cell and a separate, microvilli-bearing translucent cell. Calibration bars in (B and C): 10 µm.
Figure 3 Light responses in isolated organ of Hesse. (A) Superimposed traces of membrane voltage recording, showing depolarization elicited by brief flashes of light. (B) Light-activated inward currents measured under voltage clamp by the whole-cell patch recording technique. In both cases stimuli were delivered every minute, and the intensity of the light was increased at 0.6 log increments. Similar responses were also obtained from Joseph cells.
Addendum to:
References
- Land MF, Nilsson D-E. Animal Eyes 2001; Oxford U.K Oxford University Press
- Schneuwly S, Burg MG, Lending C, Perdew MH, Pak WL. Properties of photoreceptor-specific phospholipase C encoded by the norpA Gene of Drosophila melanogaster. J Biol Chem 1991; 266:24314 - 24319
- Fesenko EE, Kolesnikov SS, Lyubarsky AL. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature 1985; 313:310 - 313
- Gomez M, Nasi E. Activation of light-dependent potassium channels in ciliary invertebrate photoreceptors involves cGMP but not the IP3/Ca cascade. Neuron 1995; 15:607 - 618
- Martin VJ. Photoreceptors of cnidarians. Can J Zool 2002; 80:1703 - 1722
- Saló E, Pineda D, Marsal M, Gonzalez J, Gremigni V, Batistoni R. Genetic network of the eye in Platyhelminthes: expression and functional analysis of some players during planarian regeneration. Gene 2002; 287:67 - 74
- Gorman ALF, McReynolds JS. Hyperpolarizing and depolarizing receptor potentials in the scallop eye. Science 1969; 165:309 - 310
- Gomez M, Nasi E. The light-sensitive conductance of hyperpolarizing invertebrate photoreceptors: a patch-clamp study. J Gen Physiol 1994; 103:939 - 956
- Arendt D, Tessmar-Raible K, Snyman H, Dorresteijn AW, Wittbrodt J. Ciliary photoreceptors with a vertebrate-type opsin in an invertebrate brain. Science 2004; 306:869 - 871
- Purschke G, Arendt D, Hausen H, Müller MCM. Photoreceptor cells and eyes in Annelida. Arthrop Struct Dev 2006; 35:211 - 230
- Berson D, Dunn F, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 2002; 295:1070 - 1073
- Devlin PF, Kay SA. Circadian photoreception. Annu Rev Physiol 2001; 63:677 - 694
- Kardon R. Pupillary light reflex. Curr Opin Ophthalmol 1995; 6:20 - 26
- Provencio I, Jiang G, De Grip W, Hayes W, Rollag M. Melanopsin: an opsin in melanophores, brain and eye. Proc Natl Acad Sci USA 1998; 95:340 - 345
- Arendt D. Evolution of eyes and photoreceptor cell types. Int J Dev Biol 2003; 47:563 - 571
- Sekaran S, Lall GS, Ralphs KL, Wolstenholme AJ, Lucas RJ, Foster RG, et al. 2-Aminoethoxydiphenylborane is an acute inhibitor of directly photosensitive retinal ganglion cell activity in vitro and in vivo. J Neurosci 2007; 27:3981 - 3986
- Graham DM, Wong KY, Shapiro P, Frederick C, Pattabiraman K, Berson DM. Melanopsin ganglion cells use a membrane associated rhabdomeric phototransduction cascade. J Neurophysiol 2008; 99:2522 - 2532
- Putnam NH, Butts T, Ferrier DE, Furlong RF, Hellsten U, Kawashima T, et al. The amphioxus genome and the evolution of chordate karyotype. Nature 2008; 453:1064 - 1072
- Schubert M, Escriva H, Neto J-X, Laudet V. Amphioxus and tunicates as evolutionary model systems. Trends Ecol Evol 2006; 21:269 - 277
- Koyanagi M, Kubokawa K, Tsukamoto H, Shichida Y, Terakita A. Cephalochordate melanopsin: evolutionary linkage between invertebrate visual cells and vertebrate photosensitive retinal ganglion cells. Curr Biol 2005; 15:1065 - 1069
- Eakin RM, Westfall JA. Fine structure of photoreceptors in Amphioxus. J Ultra Res 1962; 6:531 - 539
- Nakao T. On the fine structure of the amphioxus photoreceptor. Tohoku J Exp Med 1964; 82:349 - 363
- Watanabe T, Yoshida M. Morphological and histochemical studies on Joseph cells of amphioxus, Branchiostoma belcheri Gray. Exp Biol 1986; 46:67 - 73
- Gomez M, Angueyra JM, Nasi E. Light-transduction in melanopsin-expressing photoreceptors of amphioxus. Proc Natl Acad Sci USA 2009; 106:9081 - 9086
- Nasi E, Gomez M, Payne R. Hoff AJ, Stavenga DG, de Grip WJ, Pugh EN. Phototransduction mechanisms in microvillar and ciliary photoreceptors of invertebrates. Molecular Mechanisms in Visual Transduction—Handbook of Biological Physics 2000; 3:Amsterdam Elsevier Science 389 - 448
- Gomez M, Angueyra JM, Nasi E. Examining the ancient phototransduction mechanisms of a primitive chordate. J Gen Physiol 2008; 132:4
- Salvini-Plawen LV, Mayr E. On the evolution of photoreceptors and eyes. Evol Biol 1977; 10:207 - 263
- Gomez M, Nasi E. Light transduction in invertebrate hyperpolarizing photoreceptors: involvement of a Go-regulated guanylate cyclase. J Neurosci 2000; 20:5254 - 5263
- Koyanagi M, Takano K, Tsukamoto H, Ohtsu K, Tokunaga F, Terakita A. Jellyfish vision starts with cAMP signaling mediated by opsin-Gs cascade. Proc Natl Acad Sci USA 2008; 105:15576 - 15580
- Darwin C. On the Origin of Species by Means of Natural Selection: or the Preservation of Favoured Races in the Struggle for Life 1859; London John Murray
- Gehring WJ, Ikeo K. Pax 6: mastering eye morphogenesis and eye evolution. Trends Genet 1999; 15:371 - 377
- Arendt D, Wittbrodt J. Reconstructing the eyes of urbilateria. Phil Trans Roy Soc Lond B 2001; 356:1545 - 1563