Abstract
Identifying the mechanisms driving adaptive radiations is key to explaining the diversity of life. The extreme reliance of spiders upon silk for survival provides an exceptional system in which to link patterns of diversification to adaptive changes in silk use. Most of the world’s 41,000 species of spiders belong to two apical lineages of spiders that exhibit quite different silk ecologies, distinct from their ancestors. Orb spiders spin highly stereotyped webs that are suspended in air and utilize a chemical glue to make them adhesive. RTA clade spiders mostly abandoned silk capture webs altogether. We recently proposed that these two clades present very different evolutionary routes of achieving the same key innovation – escape from the constraints imposed by spinning webs that contain a relatively costly type of physically adhesive cribellate silk. Here, we test the prediction that orb and RTA clade spiders are not only more diverse, but also have higher fecundity than other spiders. We show that RTA clade spiders average 23% higher fecundity and orb spiders average 123% higher fecundity than their ancestors. This supports a functional link between the adaptive escape from cribellate silk and increased resource allocation to reproduction in spiders.
Adaptative radiations explain much of the modern earth’s diversity of life.Citation1,Citation2 Yet, identifying the mechanisms driving the success of those radiations is difficult.Citation3–Citation5 Spiders provide an exceptional system in which to test links between putative adaptations and patterns of speciation because of their extreme reliance on silk. Spider webs epitomize the adaptive use of high performance biomaterials in animal architecture.Citation6,Citation7 All of the world’s 41,000+ species of spiders spin silk fibers with strength to weight ratios up to five times greater than steel. Thus, it is not surprising that the spectacular evolutionary and ecological success of spiders is generally attributed to key innovations in the production and use of different silks.Citation8–Citation12 The aerial orb webs of the Orbiculariae are one such example, which utilize a composite architecture including a framework of stiff, exceedingly strong major ampullate silk radii to suspend a highly elastic capture spiral coated with droplets of liquid glue (). The capacity of orb webs to reliably absorb the high energy impact of flying prey helped to make orbicularian spiders dominant predators of aerial insects in many ecosystems.
However, such silk production is not without cost. Silk threads are composed primarily of proteins and a single orb web can be as much as 0.1–1% of a spider’s wet body mass.Citation13 Thus, replacing lost webs from body reserves is presumably expensive. Furthermore, some silks cost more energy to spin than others. In particular, the cribellate silk used as adhesive fibers in relatively primitive spider webs functions through van der Waals interactions and physical entanglement.Citation14,Citation15 This contrasts with the chemically adhesive glycoproteins found in the aggregate glue droplets of modern orb spiders.Citation16 In a time and energy-consuming process, cribellate spiders physically comb out puffs of silk containing the hundreds to thousands of nanoscale fibrils required for adhesive function (). Consequently, spiders that utilize cribellate silk tend to show high fidelity to individual webs. In contrast, most derived spiders have either abandoned capture webs entirely or evolved chemically adhesive, aggregate glue, which allows webs to be constructed quickly and for silk to be recycled when webs are taken down and consumed.Citation17,Citation18 We recently used a total evidence based phylogeny of spiders to demonstrate that these two behavioral patterns of silk use are derived strategies that mark the two most successful clades of spiders—wandering hunter/ambushers in the RTA clade (∼22,000 species) and the orb-weaving Araneoidea (∼12,000 species). We suggested that “escape” from the constraints imposed by use of expensive cribellate silk was causally related to the latter adaptive radiations of RTA clade and Araneoidea, such that the evolutionary shifts away from the use of capture silk altogether and to chemically adhesive glue, played an important role in shaping the diversification of modern spiders. In turn, the hypothesis predicts that adaptive changes in silk use should be accompanied by increased fecundity. Here, we test this prediction by examining the correlation between changes in web use and fecundity across spiders.
Comparing Spider Fecundity
Reproductive output of spiders, measured as clutch size, generally increases with body size of spiders, both within and among species. Citation19–Citation21 Here, we present data on reproductive output from 343 species across 60 of the 105 extant families of spiders, representing all higher lineages (see appendix). Comparative data is best analyzed in a phylogenetic framework, such as independent contrasts, to control for the inflation of degrees of freedom that can occur when comparing close relatives.Citation22 However, this is not feasible for the current data because most spider phylogeny is largely unknown. Prior studies also show that the relationship between spider body size and fecundity is relatively similar regardless of the use of “raw” or phylogenetically independent data.Citation19,Citation20 Therefore, we concentrate on the phenotypic data themselves, with an understanding that Type I error may be somewhat inflated by this approach.
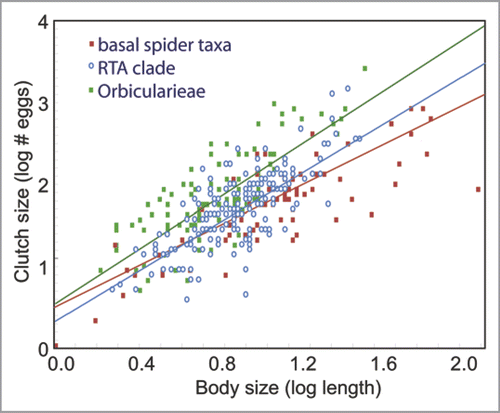
Spider length was highly correlated with spider fecundity in a regression analysis (R2 = 0.49, p < 0.00001)(). We therefore used the standardized residuals of fecundity vs. body length to compare the reproductive output of orbicularian and RTA clade spiders versus all other taxa (“outgroup”). The lineage of spiders had a highly significant effect on residual fecundity (ANOVA F2,340 = 40.4, p < 0.00001). Both RTA clade and orbicularian spiders had higher fecundity than all other spiders (23 and 123% respectively). Furthermore, orbicularians were significantly more fecund than the RTA clade ().
While other factors also contribute to reproductive output, we argue that clutch size is a good overall estimator. Energy content of eggs is similar across a broad survey of spider taxa.Citation23 Many spiders can produce more than one clutch of eggs over their lifetime, but past studies suggest that individual clutch size strongly correlates positively with number of clutches.Citation21 Finally, there is currently no consensus on the potential for an egg size-number tradeoff within clutches, with near simultaneous studies proposing evidence forCitation20 and against the hypothesis.Citation19 Thus, single clutch remains at least a reasonably accurate estimator of overall spider reproductive effort.
Fecundity and Spider Evolution
The derived predatory behaviors considered here, development of aerial webs and loss of capture webs all together, are quite different. But, both allow “escape” from dependence on expensive cribellate silk.Citation12 Moreover, we show here that this escape is significantly correlated with increased reproductive output in both clades.
The clutch sizes of orb spiders are much higher than either distant outgroup taxa or their sister lineage, the RTA clade (). The evolution of the stereotyped behaviors and the aerial frameworks of dragline silk used in the construction of orb webs may therefore have enabled access to a new source of abundant prey, flying insects, which neither hunter/ambushers nor primitive cribellate spiders can easily catch. Indeed, growing ecological data support the hypothesis that evolution has placed a premium on a fast growth life history for orb spiders. Positive, fecundity-based selection on female body size in orb spidersCitation24 necessitates high rates of prey capture.Citation25 Much of this biomass consumed by orbweavers is subsequently converted into egg production.Citation26,Citation27 While spiders are famous for their low metabolic rates,Citation28 orb spiders are exceptions. Many orb spiders have notably higher metabolismsCitation29–Citation31 and they require high rates of prey capture for survival and reproduction. Citation32 In contrast, some wolf spiders (RTA clade) and filistatids (an “outgroup” taxon in this study) can survive 200 days without food.Citation33 Thus, while the evolution of glue-coated orb webs represents a major evolutionary innovation in spiders that facilitates increased reproductive output; it may also have imposed a new set of ecological constraints that further shaped the evolution of silk use in these spiders.
Figures and Tables
Figure 1 Spiders use silk in three broadly different strategies for prey capture. (A) Many spiders spin a variety of sheet webs with relatively amorphous architectures. These webs lack stereotyped major ampullate supporting threads and utilize cribellate adhesive threads (shown here in darkfield at the bottom of the panel). Cribellate silk consists of one or more pairs of core fibers surrounded by a sheath of nanoscale fibrils physically combed into puffs by the spider. (B) Nearly all orb weaving spiders and their relatives use stereotyped web spinning behaviors and defined frameworks of dragline silk to suspend webs relatively far from substrate. Thus, the form of the web is taxonomically rather than substrate specific. Most also utilize aggregate capture silk (see text). (C) RTA clade spiders tend to stalk or ambush prey, having abandoned the use of capture silks altogether.
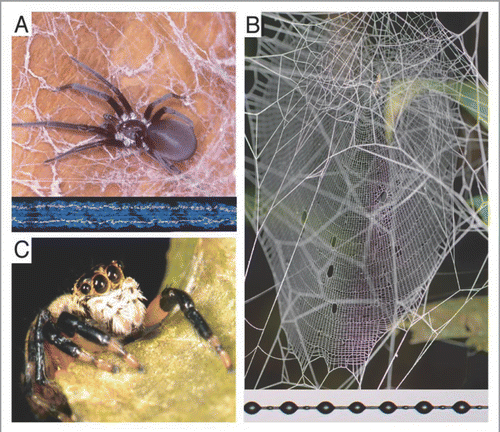
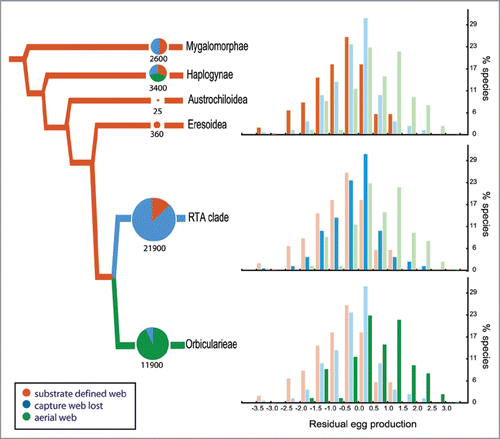
Figure 3 Relationship between fecundity and silk use by spiders. Residuals for egg production were calculated from the regression of individual clutch size on spider body length in figure 2. The graphs show histograms of residual egg production for species within each of two highly successful, apical clades of spiders compared to all other taxa of spiders. The phylogeny is summarized from Blackledge et al. (2009) and illustrates the relationship between species diversity (total size of pie charts) and silk use ecologies (colors) among spiders.
Acknowledgements
This research was supported by National Science Foundation awards #DEB-0516038 and IOS-0745379 to T.A.B. and EAR-0228699 to J.A.C., as well as by Slovenian Research Agency fellowship ARRS Z1-9799-0618-07 to I.A.
Addendum to:
References
- Wagner WL, Funk VA. Hawaiian biogeography: Evolution on a hot spot archipelago 1995; Washington, DC Smithsonian Institution Press
- Orr MR, Smith TB. Ecology and speciation. Trends Ecol Evol 1998; 13:502 - 506
- Coddington JA. Cladistic tests of adaptational hypotheses. Cladistics-Int J Willi Hennig Soc 1988; 4:3 - 22
- Schluter D. The ecology of adaptive radiation 2000; New York Oxford University Press
- Losos JB, Jackman TR, Larson A, de Queiroz K, Rodriguez-Schettino L. Contingency and determinism in replicated adaptive radiations of island lizards. Science 1998; 279:2115 - 2118
- Denny M. Physical properties of spider’s silks and their role in design of orb-webs. J Exp Biol 1976; 65:483 - 506
- Vollrath F. Spider Webs and Silks. SciAm 1992; 266:70 - 76
- Bond JE, Opell BD. Testing adaptive radiation and key innovation hypotheses in spiders. Evolution 1998; 52:403 - 414
- Blackledge TA, Coddington JA, Gillespie RG. Are three-dimensional spider webs defensive adaptations?. Ecol Lett 2003; 6:13 - 18
- Craig CL. The significance of spider size to the diversification of spider-web architectures and spider reproductive modes. Am Nat 1987; 129:47 - 68
- Coddington J. Shear WA. The monophyletic origin of the orb web. Spiders: webs, behavior and evolution 1986; Stanford Stanford University Press 319 - 363
- Blackledge TA, Scharff N, Coddington JA, Szüts T, Wenzel JW, Hayashi CY, et al. Reconstructing web evolution and spider diversification in the molecular era. Proc Natl Acad Sci USA 2009; 106:5229 - 5234
- Blackledge TA. Stabilimentum variation and foraging success in Argiope aurantia and Argiope trifasciata (Araneae: Araneidae). J Zool 1998; 246:21 - 27
- Hawthorn AC, Opell BD. van der Waals and hygroscopic forces of adhesion generated by spider capture threads. J Exp Biol 2003; 206:3905 - 3911
- Opell BD. The ability of spider cribellar prey capture thread to hold insects with different surface features. Funct Ecol 1994; 8:145 - 150
- Vollrath F, Tillinghast EK. Glycoprotein glue beneath a spider web’s aqueous coat. Naturwissenschaften 1991; 78:557 - 559
- Townley MA, Tillinghast EK. Orb web recycling in Araneus cavaticus (Araneae, Araneidae) with an emphasis on the adhesive spiral component, gabamide. J Arachnol 1988; 16:303 - 319
- Opell BD. Economics of spider orb-webs: the benefits of producing adhesive capture thread and of recycling silk. Funct Ecol 1998; 12:613 - 624
- Marshall SD, Gittleman JL. Clutch size in spiders: Is more better?. Funct Ecol 1994; 8:118 - 124
- Simpson MR. Covariation of spider egg and clutch size: the influence of foraging and parental care. Ecology 1995; 76:795 - 800
- Enders F. Clutch size related to hunting manner of spider species. Ann Entomol Soc Am 1976; 69:991 - 998
- Harvey PH, Pagel MD. The Comparative Method in Evolutionary Biology 1991; New York Oxford University Press
- Anderson JF. Energy Content of Spider Eggs. Oecologia 1978; 37:41 - 57
- Coddington JA, Hormiga G, Scharff N. Giant female or dwarf male spiders?. Nature 1997; 385:687 - 688
- Higgins L. Female gigantism in a New Guinea population of the spider Nephila maculata. Oikos 2002; 99:377 - 385
- Eberhard WG. Rates of egg-production by tropical spiders in the field. Biotropica 1979; 11:292 - 300
- Wise DH. Food limitation of the spider Linyphia marginata: experimental food studies. Ecology 1975; 56:637 - 646
- Anderson JF. Metabolic Rates of Spiders. Comparative Biochemistry and Physiology 1970; 33:51
- Anderson JF, Prestwich KN. Respiratory gas exchange in spiders. Physiol Zool 1982; 55:72 - 90
- Anderson JF. Comparative energetics of comb-footed spiders (Araneae, Theridiidae). Comp Biochem Physiol A-Physiol 1994; 109:181 - 189
- Greenstone MH, Bennett AF. Foraging strategy and metabolic rate in spiders. Ecology 1980; 61:1255 - 1259
- Venner S, Casas J. Spider webs designed for rare but life-saving catches. Proc Roy Soc B 2005; 272:1587 - 1592
- Anderson JF. Responses to starvation in the spiders Lycosa lenta Hentz and Filistata hibernalis (Hentz). Ecology 1974; 55:576 - 585
Appendix—Sources of Fecundity Data
- Amalin DM, Reiskind J, Pena JE, McSorley R. Predatory behavior of three species of sac spiders attacking citrus leafminer. J Arachnol 2001; 29:72 - 81
- Aviles L, McCormack J, Cutter A, Bukowski T. Precise, highly female-biased sex ratios in a social spider. Proc R Soc Lond Ser B-Biol Sci 2000; 267:1445 - 1449
- Bond JE, Opell BD. Systematics of the spider genera Mallos and Mexitlia (Araneae: Dictynidae). Zool J Linn Soc 1997; 119:389 - 445
- Bukowski TC, Christenson TE. Natural history and copulatory behavior of the spiny orbweaving spider Micrathena gracilis (Araneae, Araneidae). J Arachnol 1997; 25:307 - 320
- Coddington JA. The genera of the spider family Theridiosomatidae. Smithsonian Contributions to Zoology 1986; 1 - 96
- Coyle FA, Icenogle WR. Natural-History of the Californian Trapdoor Spider Genus Aliatypus (Araneae, Antrodiaetidae). J Arachnol 1994; 22:225 - 255
- Bosselaers J, Jocque R. Studies in Corinnidae: transfer of four genera and description of the female of Lessertina mutica Lawrence 1942. Trop Zool 2000; 13:305 - 325
- Boulton AM, Polis GA. Phenology and life history of the desert spider, Diguetia mojavea (Araneae, Diguetidae). J Arachnol 1999; 27:513 - 521
- Brescovit AD, Raizer J, Amaral MEC. Descriptions and notes on the genus Paradossenus in the neotropical region (Araneae, Trechaleidae). J Arachnol 2000; 28:7 - 15
- Costa FG, Perez-Miles F. Behavior, life cycle and webs of Mecicobothrium thorelli (Araneae, Mygalomorphae, Mecicobothriidae). J Arachnol 1998; 26:317 - 329
- Costa FG, Perez-Miles F. Reproductive biology of Uruguayan theraphosids (Araneae, Mygalomorphae). J Arachnol 2002; 30:571 - 587
- Coyle FA. A revison of the American funnel-web mygalomorph spider genus Euagrus (Araneae, Dipluridae). Bull Amer Mus Nat Hist 1988; 187:203 - 292
- Coyle FA, Oshields TC, Perlmutter DG. Observations on the behavior of the kleptoparasitic spider, Mysmenopsis furtiva (Araneae, Mysmenidae). J Arachnol 1991; 19:62 - 66
- Eberhard WG. Rates of egg-production by tropical spiders in the field. Biotropica 1979; 11:292 - 300
- Danielson-Francois AM. Natural history of Glenognatha emertoni (Araneae, Tetragnathidae): Mating behavior and sperm release in a haplogyne. J Arachnol 2006; 34:387 - 398
- Deng LL, Dai JY, Cao H, Xu MQ. Effects of an organophosphorous insecticide on survival, fecundity and development of Hylyphantes graminicola (Sundevall) (Araneae: Linyphiidae). Environ Toxicol Chem 2006; 25:3073 - 3077
- Doran NE, Richardson AMM, Swain R. The reproductive behaviour of the Tasmanian cave spider Hickmania troglodytes (Araneae: Austrochilidae). J Zool 2001; 253:405 - 418
- Downes MF. The life-hisotry of Badumna candida (Araneae, Amaurobioidea). Aust J Zool 1993; 41:441 - 466
- Edwards RL, Edwards EH, Edwards AD. Observations of Theotima minutissimus (Araneae, Ochyroceratidae), a parthenogenetic spider. J Arachnol 2003; 31:274 - 277
- Forster RR, Platnick NI. A review of the archaeid spiders and their relatives, with notes on the limits of the superfamily Palpimanoidea (Arachnida, Araneae). Bull Amer Mus Nat Hist 1984; 178:1 - 106
- Griswold CE, Meikle-Griswold T. Archaeodictyna ulova, new species (Araneae: Dictynidae), a remarkable kleptoparasite of group-living eresid spiders (Stegodyphus spp., Araneae: Eresidae). Am Mus Novit 1987; 2897:1 - 11
- Gundermann JL, Horel A, Roland C. Costs and benefits of maternal care in a subsocial spider, Coelotes terrestris. Ethology 1997; 103:915 - 925
- Hormiga G. A revision and cladistic analysis of the spider family Pimoidae (Araneoidea: Araneae). Smithson Contrib Zool 1994; 549:1 - 104
- Kaston BJ. Spiders of Connecticut. State Geological and Natural History Survey of Connecticut 1981;
- Kim KW, Roland C, Horel A. Functional value of matriphagy in the spider Amaurobius ferox. Ethology 2000; 106:729 - 742
- Fischer ML, Vasconcellos-Neto J. Parameters affecting fecundity of Loxosceles intermedia Mello-Leitao 1934 (Araneae, Sicariidae). J Arachnol 2005; 33:670 - 680
- Grismado CJ. A taxonomic revision of the spider genus Ariadna audouin, 1826 in Argentina and Chile, with the description of five new species (Arachnida, Araneae, Segestriidae). Zoosystema 2008; 30:333 - 360
- Griswold CE. A revision and phylogenetic analysis of the spider subfamily Phyxelidinae (Araneae, Amaurobiidae). Bull Amer Mus Nat Hist 1990; 3 - 206
- Guarisco H. Description of the egg sac of Mimetus notius (Araneae, Mimetidae) and a case of egg predation by Phalacrotophora epeirae (Diptera, Phoridae). J Arachnol 2001; 29:267 - 269
- Huber BA. Notes on the neotropical spider genus Modisimus (Pholcidae, Araneae), with descriptions of thirteen new species from Costa Rica and neighboring countries. J Arachnol 1998; 26:19 - 60
- Jocque R. A generic revision of the spider family Zodariidae (Araneae). Bull Amer Mus Nat Hist 1991; 1 - 160
- Cazier MA, Mortenson MA. Analysis of the habitat, web design, cocoon and egg sacs of the tube weaving spider Diguetia canities (McCook), (Araneae, Diguetidae). Bull South Calif Acad Sci 1962; 61:65 - 88
- Coyle FA, Meigs TE. Two new species of kleptoparasitic Mysmenopsis (Araneae, Mysmenidae) from Jamaica. J Arachnol 1989; 17:59 - 70
- Calderón R, Garrido M, Pinto C. Etapas del crecimiento de Acanthogonatus franckii Karsch, 1880 (Araneae: Nemesiidae). Rev Chil Entomol 1990; 18:19 - 24
- Bennett RG. Systematics and natural history of Wadotes (Araneae, Agelenidae). J Arachnol 1987; 15:91 - 128
- Baerg WJ. Tarantula studies. J NY Entomol Soc 1938; 46:31 - 43
- Bentzien MM. Biology of the spider Diguetia imperiosa. The Pan-Pac Entomol 1973; 49:110 - 123
- Bristowe WS. The comity of spiders 1939; London The Ray Society
- Forster RR, Forster LM. Spiders of New Zealand 1999; Dunedin University of Otago Press
- Ihara Y. Cybaeus jinsekiensis n. sp., a spider species wtih protogynous maturation and mating plugs (Araneae: Cybaeidae). Acta Arachnologica 2006; 55:5 - 13
- Huff RP, Coyle FA. Systematics of Hypochilus sheari and Hypochilus coylei, two Southern Appalachian lampshade spiders (Araneae, Hypochilidae). J Arachnol 1992; 20:40 - 46
- Girault AA. Standards of the number of eggs laid by spiders—I (Arach.). Entomol News 1911; 22:461 - 462
- Girault AA. Standards of the number of eggs laid by spiders (Aran.)—III. Entomol News 1914; 25:66 - 67
- Gertsch WJ, Platnick NI. A revision of the spider family Mecicobothriidae (Araneae, Mygalomorphae). Am Mus Novit 1979; 2687:1 - 32
- Paz N. Aspectos de la biología reproductiva de Linothele megatheloides (Araneae: Dipluridae). J Arachnol 1993; 21:40 - 49
- de Andrade RMG, Lourenço WR, Tambourgi DV. Comparison of the fertility between Loxosceles intermedia and Loxosceles laeta spiders (Araneae, Sicariidae). J Arachnol 2 2000; 28:245 - 247
- Fergusson IC. Natural history of the spider Hypochilus thorelli Marx (Hypochilidae). Psyche 1972; 79:179 - 199
- Levi HW. Orb-weaving spiders Actinosoma, Spilasma, Micrepeira, Pronous and four new genera (Araneae: Araneidae). Bull Mus Comp Zool 1995; 154:153 - 211
- Labarque FM, Ramírez MJ. Description of the female of Drymusa serrana Goloboff & Ramírez, 1991 (Araneae: Drymusidae) with notes on its biology. Zootaxa 2007; 1580:27 - 33
- Levi HW. The neotropical orb-weaving spiders of the genera Wixia, Pozonia and Ocrepeira (Araneae: Araneidae). Bull Mus Comp Zool 1993; 153:47 - 141
- Levi HW. The Neotropical and Mexican species of the orb-weaver genera Araneus, Dubiepeira new genus, and Aculepeira (Araneae: Araneidae). Bull Mus Comp Zool 1991; 152:167 - 315
- Levi HW. The bolas spiders of the genus Mastophora (Araneae: Araneidae). Bull Mus Comp Zool 2003; 157:309 - 382
- Levi HW. The American orb-weaver genera Larinia, Cercidia and Mangora north of Mexico (Araneae, Araneidae). Bull Mus Comp Zool 1975; 147:101 - 135
- Levi HW. The orb-weaver genera Araniella and Nuctenea (Araneae: Araneidae). Bull Mus Comp Zool 1974; 146:291 - 316
- Marshall SD, Gittleman JL. Clutch size in spiders: Is more better?. Funct Ecol 1994; 8:118 - 124
- Marshall SD, Uetz GW. The growth and maturation of a giant spider: Theraphosa leblondi (Latreille, 1804) (Araneae, Theraphosidae). Rev Arachnol 1993; 10:93 - 103
- Lubin YD, Eberhard WG, Montgomery GG. Webs of Miagrammopes (Araneae: Uloboridae) in the neotropics. Psyche 1978; 85:1 - 23
- Maddison WP. Pelegrina franganillo and other jumping spiders formerly placed in the genus Metaphidippus (Araneae: Salticidae). Bull Mus Comp Zool 1996; 154:215 - 368
- Main BY. Biology of aganippine trapdoor spiders (Mygalomorphae: Ctenizidae). Aust J Zool 1957; 5:402 - 473
- McLay CL, Hayward TL. Reproductive biology of the intertidal spider Desis marina (Araneae: Desidae) on a New Zealand rocky shore. J Zool 1987; 211:357 - 372
- Miyashita T. Growth, egg production and population density of the spider, Nephila clavata in relation to food conditions in the field. Res Popul Ecol 1986; 28:135 - 150
- Miyashita K. Life cycle of Oecobius annulipes Lucas (Araneae: Oecobiidae) under indoor conditions and the effect of photoperiod on nymphal development. Acta Arachnologica 1992; 41:5 - 10
- Nielsen E. The Biology of Spiders. Vol. 1 1931; Copenhagen, Denmark Levin & Munksgaard
- Opell BD, Beatty JA. The nearctic Hahniidae (Arachnida: Araneae). Bull Mus Comp Zool 1976; 147:393 - 433
- Opell BD, Berger AM, Shaffer RS. The body size of the New Zealand orb-weaving spider Waitkera waitakerensis (Uloboridae) is directly related to temperature and affects fecundity. Invertebr Biol 2007; 126:183 - 190
- Prenter J, Elwood RW, Montgomery WI. Sexual size dimorphism and reproductive investment by female spiders: A comparative analysis. Evolution 1999; 53:1987 - 1994
- Salomon M, Lubin Y. Cooperative breeding increases reproductive success in the social spider Stegodyphus dumicola (Araneae, Eresidae). Behav Ecol Sociobiol 2007; 61:1743 - 1750
- Edwards RL, Edwards AD. Life history and ecology of the armored spider Monoblemma muchmorei (Araneae, Tetrablemmidae). J Arachnol 2006; 34:599 - 609
- Reichling SB, West RC. A new genus and species of Theraphosid spider from Belize (Araneae, Theraphosidae). J Arachnol 1996; 24:254 - 261
- Schick RX. The crab spiders of California (Araneidae, Thomisidae). Bull Amer Mus Nat Hist 1965; 129:1 - 180
- Punzo F, Henderson L. Aspects of the natural history and behavioural ecology of the tarantula spider Aphonopelma hentzi (Girard, 1854) (Orthognatha, Theraphosidae). Bull Br Arachnol Soc 1999; 11:121 - 128
- Platnick NI, Schwendinger PJ, Steiner H. Three new species of the spider genus Liphistius (Araneae, Mesothele) from Malaysia. Am Mus Novit 1997; 3209:1 - 13
- Platnick NI. A revision of the spider genus Segestrioides (Araneae, Diguetidae). Am Mus Novit 1989; 2940:1 - 9
- Platnick NI. A revision of the South American spider genus Trachelpachys (Araneae, Clubionidae). Am Mus Novit 1975; 2589:1 - 25
- Ono H. Spiders of the genus Heptathela (Araneae, Liphistiidae) from Vietnam with notes on their natural history. J Arachnol 1999; 27:37 - 43
- Schneider JM, Salomon M, Lubin Y. Limited adaptive life-history plasticity in a semelparous spider, Stegodyphus lineatus (Eresidae). Evol Ecol Res 2003; 5:731 - 738
- Suter RB. Determinants of fecundity in Frontinella pyramitela (Araneae, Linyphiidae). J Arachnol 1990; 18:263 - 269
- Watanabe T. Life history and seasonal change in the frequency of dimorphic stabilimenta of the orbweb spider, Octonoba sybotides (Uloboridae). Acta Arachnologica 2000; 49:1 - 12
- West HP, Toft S. Last-male sperm priority and the mating system of the haplogyne spider Tetragnatha extensa (Araneae: Tetragnathidae). J Insect Behav 1999; 12:433 - 450
- Locht A, Yanez M, Vazquez I. Distribution and natural history of Mexican species of Brachypelma and Brachypelmides (Theraphosidae, Theraphosinae) with morphological evidence for their synonymy. J Arachnol 1999; 27:196 - 200
- Schutz D, Taborsky M. Mate choice and sexual conflict in the size dimorphic water spider Argyroneta aquatica (Araneae, Argyronetidae). J Arachnol 2005; 33:767 - 775
- Schwendinger PJ. Two new species of the arboreal trapdoor spider genus Sason (Araneae: Barychelidae) from Southeast Asia. Raffles Bull Zool 2003; 51:197 - 207
- Smith DR. Notes on the reproductive biology and social behavior of two sympatric species of Philoponella (Araneae, Uloboridae). J Arachnol 1997; 25:11 - 19
- Stiles GJ, Coyle FA. Habitat distribution and life history of species in the spider genera Theridion, Rugathodes and Wamba in the Great Smoky Mountains National Park (Araneae, Theridiidae). J Arachnol 2001; 29:396 - 412
- Vetter RS, Cokendolpher JC. Homalonychus theologus (Araneae, Homalonychidae): Description of eggsacs and a possible defensive posture. J Arachnol 2000; 28:361 - 363
- Vincent LS. The natural history of the California turret spider Atypoides riversi (Aranea, Antrodiaetidae)—demographics, growth-rates, survivorship and longevity. J Arachnol 1993; 21:29 - 39
- Wang XP. A generic-level revision of the spider subfamily Coelotinae (Araneae, Amaurobiidae). Bull Amer Mus Nat Hist 2002; 3 - 150
- Shear WA. The spider family Oecobiidae in North America, Mexico and the West Indies. Bull Mus Comp Zool 1970; 140:129 - 164
- Coyle FA. The Mygalomorph Spider Genus Microhexura. Bull Amer Mus Nat Hist 1981; 170:64 - 75
- Valerio CE. Spitting spiders (Araneae, Scytodidae, Scytodes) from Central America. Bull Amer Mus Nat Hist 1981; 170:80 - 89
- Lowrie C, Dondale CD. A Revision of the nigra Group of the Genus Pardosa in North America (Araneae, Lycosidae). Bull Amer Mus Nat Hist 1981; 170:125 - 139