Abstract
Flowers interact simultaneously with a variety of insect visitors, including mutualistic pollinators and antagonists such as florivores, nectar robbers and pollinator predators. The plant epidermis produces a range of structures, such as conical or papillate cells, that can help mutualists to grip the flower, while a variety of other structures, such as slippery wax crystals on the flowers or on the stems leading to them, are able to deter non-beneficial insects or behaviours. Modification of the floral surface can also aid pollination in unusual ways in some highly specialised interactions. In the case of the trap-flowers in species of Arisaema, conical cells aid pollination by being present on the spathe surface, but here they are modified in such a way as to decrease the pollinating insect’s grip. We discuss a variety of these floral structural features that influence insect stability on the plant.
Structures on the Floral Surface that Improve Pollinator Grip
The interactions between plants and animals can be either mutually beneficial or detrimental for one of the parties. Flowers offer a range of rewards from sugary nectar to protein-rich pollen, and thus are a rich resource not only for pollinating mutualists, but also for antagonists such as florivores, predators of pollinators and nectar robbers. Therefore a flower relying on insect pollinators must both attract and repulse insects simultaneously but selectively. The flower surface is the initial point of physical contact between a plant and a flying insect visitor. The floral epidermis often contains specific structures that facilitate these different outcomes. One of the structures frequently found on the petal epidermis, in approximately 80% of 200 plant species analysed, is that of conical or papillate cells.Citation1 These cells have been shown to affect the flower in a number of ways relevant to attracting insect pollinators, including modification of floral color and temperature.Citation2–Citation6 These cells also appear to play an important role in determining how efficiently the flower is handled during foraging by some insect pollinators. In Antirrhinum, the mixta mutant, which lacks conical petal epidermal cells, was shown to be less attractive to bumblebee pollinators than wild type flowers.Citation3 While both flower color and temperature are affected by the lack of conical cells in the mixta mutant, bumblebees did not discriminate between artificial flowers altered in these parameters to the degree achieved by epidermal cell shape.Citation4,Citation5 Our recent laboratory tests found that bumblebees failed to show the preference for conical cells observed in the field if Antirrhinum flowers were held open and at a horizontal angle, where the bees could land and forage without difficulty. However, when the flowers were left closed and in their usual vertical position, bees were observed to have difficulty opening and entering the flowers and the preference for conical-celled flowers was again observed.Citation6 Biomimetic replication of the floral surface, presented to foraging bumblebees at either flat or steep angles, indicated that the presence of conical cells aids bumblebee grip and increases foraging efficiency.Citation7 However, conical petal cells are found more frequently than initial floral angle would suggest—many flowers that are simple to handle and are held at flat or shallow angles also have conical epidermal cells. While floral color, wettability and temperature, which are also affected by the presence of conical cells, could be more important in these particular flowers than in Antirrhinum, it is also possible that other pollinator slip factors, such as wind; the landing of the pollinator, leading to bending and shaking of the flower; or decreased traction due to rain may cause the increased grip provided by conical cells to be advantageous to many flowers. We suggest that this ‘grip’ function of floral epidermal cells will be important for a wide range of insect pollinators. While it is evident why pollinators such as bumblebees, which have relatively small tarsal adhesive pads for their size,Citation8 should rely on grip by their interlocking claws, other flower visitors with relatively larger tarsal adhesive structures such as flies and honeybees should not have a significant problem with smooth flowers. However, contamination of the tarsae by pollen grains might inhibit their adhesive function, so that interlocking by claws becomes more important.
Structural Modifications of Flower Stems that Deter Nectar Thieves
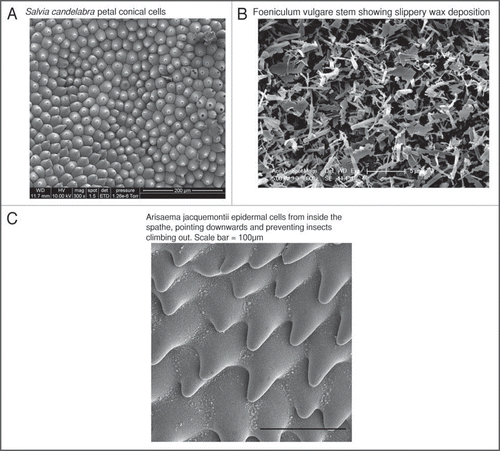
The pollination relationship between plants and insects is not always simply mutually beneficial. Both sides on occasion cheat, and some plants have modified surface morphology that minimises the consequences of this. One example of insect cheating is robbing of nectar without providing a pollination service. Many plants have defensive structures that limit access to the flowers for potential nectar thieves. As a result of their near-ubiquitous occurrence in most habitats,Citation9 ants show a range of interactions with plants. While there is evidence that some plant species are ant-pollinated,Citation10 most are pollinated by flying insects, and for these species ants behave primarily as nectar thieves. This can be detrimental to plant fitness in several ways. Nectar-robbing reduces the amount of reward a plant has available for genuine pollinators, potentially resulting in fewer pollinator visits and reduced reproductive output. Ants can also damage flowersCitation11 or reduce pollen fertility,Citation12 and have been shown to reduce the rate of visits from flying insects, either by attacking pollinators that land or just by their presence.Citation13,Citation14 Plants have developed a range of mechanical defences against nectar-robbing ants. Plumbago auriculata, for example, has glandular trichomes on the calyx which exude a sticky substance, acting as a barrier against crawling insects. This means that ants do not have access to the interior of the flower, while flying insects do.Citation15 The epidermis can also be modified to deter ants. While structures on the petal epidermis positively aid the grip of insect feet, the stem leading to the flowers does the opposite in many species. One example is displayed by Salvia candelabra. While the petal epidermis produces conical cells (), which will provide grip for flying insect pollinators, the top of the stem leading to the flower is covered by epicuticular wax crystals (see ). Flower stems covered by wax crystals are found in many different plant families. This wax crystal bloom makes gripping the flower stem difficult for insects such as ants that might rob flowers.Citation16,Citation17 Wax particles can also break off and clog the tarsal adhesive structures, further decreasing insect grip.Citation18 As the waxy surfaces are mostly restricted to the stems, many of the other proposed functions of epicuticular wax crystals (protection against excessive radiation,Citation19 reduction of transpiration,Citation20 inhibition of pathogen attachmentCitation21 and self-cleaning abilityCitation22) are implausible here, suggesting that waxy stems have evolved primarily as climbing barriers against nectar-robbing ants.
Modification of the Epidermis in Trap-Flowers
Cheating is not restricted to animals, but is also practiced by plants. For example, some plants cheat by advertising nonexistent rewards, or by trapping and even sometimes killing their pollinators. The genus Arisaema produces kettle-trap inflorescencesCitation18 (consisting of a unisexual inflorescence surrounded by a cup-like spathe) which cheat in this way. While male inflorescences trap and then release the fungus gnats attracted by the strong floral volatiles, female inflorescences trap but do not release the gnats. Having provided a pollination service, the gnats die within the female inflorescence. As with more conventionally pollinated flowers, Arisaema has modified epidermal structures that aid pollination. Analysis of the surface structures of the spathe by scanning electron microscopy (SEM) showed that all of the species studied produced a wax crystal bloom similar to those found on the stem of Salvia candelabra.Citation18 While the spathe of most Arisaema species had flat, smooth epidermal cells, two species (A. utile and A. jacquemontii) produce modified conical cellsCitation18 that point straight down into the kettle-trap (). Similar cells have been found in some carnivorous pitfall traps such as Nepenthes, Darlingtonia, Sarracenia and Cephalotus, and it has been hypothesized that these overlapping downward pointing ‘tooth’ cells might make the slide of the insect into the trap very easy, but could then block any escape upwards.Citation23 Vogel and MartinsCitation18 suggest that the presence of these cells is a trait that aids in insect capture, as downward-pointing conical cells are also found in kettle traps of the related genus Arum, which capture more ‘alert’ insects than fungus gnats.Citation24,Citation25 The presence of trapped insects may also provide an incentive for more predatory insects to enter the trap, potentially damaging the flower in the process. For example, in the genus Ceropegia, which also produces trap flowers, evidence has been found that ants prey on the trapped pollinators.Citation26 Modification of the epidermis appears to prevent this in Arisaema, where a thick layer of epicuticular wax is observed around the outside of the base of the inflorescence below the spathe (Whitney H.M., personal observation). Flowers therefore appear to produce a range of epidermal structures within and around an inflorescence that either optimise pollination or discourage damaging non-pollinating visitors.
Figures and Tables
Acknowledgements
H.M.W. gratefully acknowledges a Lloyd’s of London Tercentenary foundation fellowship and a British Ecological Society Small Projects Grant. The original body of this work was funded by Natural Environment Research Council grant NE/C000552/1 to B.J.G. and L.C. H.M.W. would also like to thank Mr. T. Marden at Shady Plants nursery (Stroud, UK) for information regarding Arisaema species.
Addendum to:
References
- Kay QON, Daoud HS, Stirton CH. Pigment distribution, light reflection and cell structure in petals. Bot J Linn Soc 1981; 83:57 - 84
- Noda K, Glover BJ, Linstead P, Martin C. Flower colour intensity depends on specialized cell shape controlled by a Myb-related transcription factor. Nature 1994; 369:661 - 664
- Comba L, Corbet SA, Hunt H, Outram S, Parker JS, Glover BJ. The role of genes influencing the corolla in pollination of Antirrhinum majus. Plant Cell Environ 2000; 23:639 - 647
- Dyer AG, Whitney HM, Arnold SEJ, Glover BJ, Chittka L. Bees associate warmth with floral colour. Nature 2006; 442:525
- Dyer AG, Whitney HM, Arnold SEJ, Glover BJ, Chittka L. Mutations perturbing petal cell shape and anthocyanin synthesis influence bumblebee perception of Antirrhinum majus flower colour. Arthropod-Plant Interact 2007; 1:45 - 55
- Gorton HL, Vogelmann TC. Effects of epidermal cell shape and pigmentation on optical properties of Antirrhinum petals at visible and ultraviolet wavelengths. Plant Physiol 1996; 112:879 - 888
- Whitney HM, Chittka L, Bruce TJA, Glover BJ. Conical epidermal cells allow bees to grip flowers and increase foraging efficiency. Curr Biol 2009; 19:948 - 953
- Pouvreau A. Morphology and histology of tarsal glands in bumble bees of the genera Bombus, Pyrobombus and Megabombus. Can J Zool 1991; 69:866 - 872
- Höldobler B, Wilson EO. The ants 1990; Cambridge, MA Belknap-Harvard University Press
- Galen C. The effects of nectar thieving ants on seedset in floral scent morphs of Polemonium viscosum. Oikos 1983; 41:245 - 249
- Galen C, Butchart B. Ants in your plants: effects of nectar-thieves on pollen fertility and seed-siring capacity in the alpine wildflower, Polemonium viscosum. Oikos 2003; 101:521 - 528
- Beattie AJ, Turnbull C, Knox RB, Williams EG. Ant inhibition of pollen function: a possible reason why ant pollination is rare. Am J Bot 1984; 71:421 - 426
- Junker R, Chung AYC, Bluthgen N. Interaction between flowers, ants and pollinators: additional evidence for floral repellence against ants. Ecol Res 2007; 22:665 - 670
- Tsuji K, Hasyim A, Nakamura H, Nakamura K. Asian weaver ants, Oecophylla smaragdina, and their repelling of pollinators. Ecol Res 2004; 19:669 - 673
- Whitney HM, Glover BJ. Coevolution: plant-insect. Enyclopedia of Life Sciences 2007;
- Federle W, Maschwitz U, Fiala B, Riederer M, Höldobler B. Slippery ant-plants and skilful climbers: selection and protection of specific ant partners by epicuticular wax blooms in Macaranga (Euphorbiaceae). Oecologia 1997; 112:217 - 224
- Harley R. Huxley CR, Cutler DF. The greasy pole syndrome. Ant-plant interactions 1991; Oxford Oxford University Press, 430 - 433
- Vogel S, Martens J. A survey of the function of the lethal kettle traps of Arisaema (Araceae), with records of pollinating fungus gnats from Nepal. Bot J Linn Soc 2000; 133:61 - 100
- Clark JB, Lister GR. Photosynthetic action spectra of trees 2. The relationship of cuticle structure to the visible and UV spectral properties of needles from four coniferous species. Plant Physiol 1975; 55:407 - 413
- Jeffree CE, Johnson RPC, Jarvis PG. Epicuticular wax in the stomatal antechamber of Sitka spruce and its effects on the diffusion of water vapour and carbon dioxide. Planta 1971; 98:1 - 10
- Holloway PJ. Chemistry of leaf waxes in relation to wetting. J Sci Food Agric 1969; 20:124 - 128
- Barthlott W, Neinhuis C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997; 202:1 - 8
- Juniper BE, Robins RJ, Joel DM. The Carnivorous Plants 1989; London, San Diego Academic Press,
- Yeo PF. Miscellaneous notes on pollination and pollinators. J Nat His 1972; 6:667 - 686
- Dormer KJ. The truth about pollination in Arum. New Phytol 1960; 59:298 - 301
- Ollerton J. Fly trapping in Ceropegia flowers-evidence of ant-predation of pollinators. Asklepios 1999; 77:31 - 32