Abstract
Various receptors navigate through the endocytic recycling compartment (ERC) on route to the plasma membrane. They are transported through recycling endosomes that emanate from the ERC that display distinct tubular morphology. A key question in the field is how the trafficking via these endosomes is regulated and how regulatory proteins such as Rab35, Rab8, Arf6 and EHD1 control this trafficking. Recent studies point to the protein MICAL-L1 as a major scaffold for these regulators. MICAL-L1 not only localizes to these tubular recycling endosomes and regulates trafficking, but it also controls the localization of EHD1 and Rab8 to these structures. It also connects its associated membranes to the motor proteins dynein and kinesin through its binding partner, CRMP2. Our recent study promotes MICAL-L1 as a Rab35 effector, where Rab35, both directly and indirectly through Arf6, controls the localization of MICAL-L1 and Rab8 to tubular membranes. We find that MICAL-L1 is a multi-tasking scaffold connecting various proteins to recycling endosomes for efficient trafficking.
Membrane trafficking controls the localization of receptors involved in signaling, and uptake of nutrients and lipids. Receptors are internalized from the plasma membrane to a sorting station called the early endosome; subsequently the internalized proteins either proceed to a degradative compartment or are recycled back to the plasma membrane by slow or fast recycling pathways.Citation1 Various GTPases including members of the Rab and Arf family proteins, as well as the C-terminal Eps15 Homology (EHD) ATPases regulate trafficking at distinct and overlapping transport steps. A ubiquitously expressed EHD family member, EHD1, localizes to tubular membranes that comprise part of the slow recycling pathway.Citation2 EHD1 functions in coordination with Arf6 and various Rabs to control membrane and receptor trafficking. Our interest in EHD1 has spurred the identification of several interaction partners such as Rab11-FIP2,Citation3 Rabenosyn-5Citation4 and Molecule interacting with CasL-Like1 (MICAL-L1),Citation5 all of which are also Rab effector proteins. Among these Rab effectors, we demonstrated that MICAL-L1 is a key player in the control of the slow recycling pathway by serving as a membrane hub to recruit regulators to the recycling endosomes.Citation5-Citation7
MICAL-L1 was identified initially as a potential effector for Rab8, Rab10, Rab13, Rab15, Rab35 and Rab36, through its binding to GTP-locked Rab mutants in a selective yeast two-hybrid screen.Citation8 Based on this study it was proposed that MICAL-L1 potentiates a crucial role in membrane trafficking.
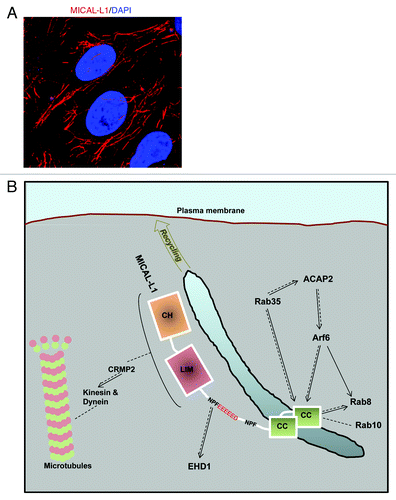
Using a proteomic screen to identify proteins that bind to EH-domain of EHD1, our studies identified MICAL-L1 as an EHD1 interaction partner.Citation5 One of the hallmarks of MICAL-L1 is that it localizes to a striking array of tubular membranes that overlap closely with those to which EHD1 localizes (). MICAL-L1 consists of a calponin homology (CH) domain, a Lin11_Isl-1_Mec-3 (LIM) domain and a coiled-coil (CC) region along with two aspargine-proline-phenylalanine (NPF) motifs. The first MICAL-L1 NPF motif is followed by acidic residues and binds to the EH domain of EHD1, whereas its CC region localizes to tubular membranes and is needed for binding to Rab8.Citation5,Citation9 Rab8 also localizes to tubular membranes and regulates receptor recycling and secretion.Citation10 MICAL-L1 not only serves as a link between EHD1 and Rab8, but its depletion prevents the association of both EHD1 and Rab8 with tubular membranes.Citation5 MICAL-L1-depletion also impairs transferrin receptor and integrin recycling. These studies highlight the role of MICAL-L1 as a trafficking regulator.
Figure 1. (A) MICAL-L1 localizes to tubular recycling endosomes. HeLa cells grown on coverslips were fixed, permeabilized and incubated with anti-MICAL-L1 antibody. Cells were then incubated with Alexa-Fluor-conjugated anti-mouse antibody and DAPI. (B) Model depicting MICAL-L1 interaction partners and functional interplay with various trafficking regulators. Through its CH and LIM domains, MICAL-L1 binds to CRMP2 to modulate trafficking via kinesin and dynein motor proteins. MICAL-L1 associates with EHD1 through its first NPF motif that is followed by a cluster of acidic residues, thus controlling the localization of EHD1 to tubular membranes. MICAL-L1, a Rab35 effector, binds to Rab35, Rab8, Rab10, and Arf6 through its CC region. Rab35 regulates MICAL-L1 localization to tubular membranes and also controls Arf6 activation through ACAP2. Arf6 controls MICAL-L1 localization to tubular endosomes and in turn, MICAL-L1 controls Rab8 localization. Dotted lines indicate a direct association between proteins. Solid lines indicate a functional interplay between the proteins, where arrowheads point toward the protein being regulated. Diagram is not drawn to scale.
We next found that MICAL-L1 links its associated membranes to motor proteins. In another proteomic screen to identify proteins that bind to the CH and LIM domains of MICAL-L1, we identified the Collapsin Response Mediator Protein-2 (CRMP2).Citation6 CRMP2 is involved in axonal growth, interacts with tubulin dimers and kinesin and negatively regulates dynein motor proteins.Citation11,Citation12 One crucial question is how cytoplasmic vesicles from the cell periphery are transported to the recycling compartment. This study showed that the interaction between MICAL-L1 and CRMP2 regulates the transport of MICAL-L1/EHD1 containing vesicles through binding to dynein and microtubules.Citation6
Although categorized as a Rab effector, MICAL-L1 actually recruits Rab8 to membranes. Thus it remained unclear whether MICAL-L1 also acts as a typical effector for other Rab proteins (i.e., whether it is in turn recruited by a Rab protein to membranes). As both GTP-locked Rab10 and Rab35 bind to the CC region of MICAL-L1, we hypothesized that they are regulators of MICAL-L1.Citation7 Although Rab10 controls receptor recycling,Citation13 neither overexpression of Rab10 wild-type or dominant-active mutants, nor its knock-down affected MICAL-L1 localization to tubular membranes. Surprisingly, overexpression of wild-type Rab35 or its dominant-active mutant impaired the membrane tubule association of MICAL-L1. Moreover, Rab35-depletion increased the level of MICAL-L1 associated with tubular endosomes, implicating MICAL-L1 as an effector of Rab35.Citation7 Rab35 is an important Rab that regulates receptor recycling, exosome secretion, phagosome formation during phagocytosis, actin cytoskeleton organization, cytokinesis, and controls T-cell antigen receptor localization to the immune synapse.Citation14-Citation16 Thus, MICAL-L1 forges a link between the tubular membranes and Rab35, a Rab with multiple cellular functions.
Rab35 also indirectly regulates Arf6 through its activating protein, ACAP2.Citation16-Citation18 Arf6 regulates clathrin-independent internalization and recycling of membranes and localizes to tubular membranes containing EHD1/MICAL-L1.Citation19,Citation20 Accordingly, three GTPases, Rab35, Rab8 and Arf6 all associate with tubular membranes containing MICAL-L1, and we addressed the sequential manner by which these GTPases regulate recycling.
While overexpression of MICAL-L1 alone increased the tubular localization of Rab8, co-expression with Rab35 dissociated Rab8 from membrane tubules.Citation7 This suggests that Rab35 regulates Rab8 localization through its interaction with MICAL-L1. Overexpression of wild-type Arf6 did not affect the tubular localization of Rab8, but its dominant-active mutant caused Rab8 to accumulate within Arf6/PI(4,5)P2-containing vacuoles. These results indicate that Arf6 determines the morphology of membrane structures to which Rab8 localizes. We also found that MICAL-L1 interacts with Arf6 through its CC region, suggesting that Arf6-mediated effects on Rab8 occur, at least in part, via MICAL-L1. Depletion of Arf6 by SiRNA treatment significantly reduced the tubular localization of MICAL-L1, indicating that Arf6 regulates MICAL-L1, which in turn regulates Rab8. Overall, MICAL-L1 is an effector of Rab35, and the latter functions upstream of MICAL-L1 and Arf6 in controlling receptor recycling. In turn, both Arf6 and MICAL-L1 regulate Rab8.
On the one hand, MICAL-L1 recruits EHD1 and Rab8 to tubular membranes and connects membranes associated with it to motor proteins (). On the other hand, it is controlled by both Rab35 and Arf6. Overall, these studies emphasize the role of MICAL-L1 as a membrane-hub that connects various trafficking effectors to regulate recycling.
Acknowledgments
We gratefully acknowledge the support of NIH grants R01GM087455 and P20 RR018759 (N.N. and S.C.). We also thank James Reinecke and Dawn Katafiasz for contributing to the figure.
References
- Jovic M, Sharma M, Rahajeng J, Caplan S. The early endosome: a busy sorting station for proteins at the crossroads. Histol Histopathol 2010; 25:99 - 112; PMID: 19924646
- Naslavsky N, Caplan S. EHD proteins: key conductors of endocytic transport. Trends Cell Biol 2011; 21:122 - 31; http://dx.doi.org/10.1016/j.tcb.2010.10.003; PMID: 21067929
- Naslavsky N, Rahajeng J, Sharma M, Jovic M, Caplan S. Interactions between EHD proteins and Rab11-FIP2: a role for EHD3 in early endosomal transport. Mol Biol Cell 2006; 17:163 - 77; http://dx.doi.org/10.1091/mbc.E05-05-0466; PMID: 16251358
- Naslavsky N, Boehm M, Backlund PS Jr., Caplan S. Rabenosyn-5 and EHD1 interact and sequentially regulate protein recycling to the plasma membrane. Mol Biol Cell 2004; 15:2410 - 22; http://dx.doi.org/10.1091/mbc.E03-10-0733; PMID: 15020713
- Sharma M, Giridharan SS, Rahajeng J, Naslavsky N, Caplan S. MICAL-L1 links EHD1 to tubular recycling endosomes and regulates receptor recycling. Mol Biol Cell 2009; 20:5181 - 94; http://dx.doi.org/10.1091/mbc.E09-06-0535; PMID: 19864458
- Rahajeng J, Giridharan SS, Naslavsky N, Caplan S. Collapsin response mediator protein-2 (Crmp2) regulates trafficking by linking endocytic regulatory proteins to dynein motors. J Biol Chem 2010; 285:31918 - 22; http://dx.doi.org/10.1074/jbc.C110.166066; PMID: 20801876
- Rahajeng J, Giridharan SS, Cai B, Naslavsky N, Caplan S. MICAL-L1 is a tubular endosomal membrane hub that connects Rab35 and Arf6 with Rab8a. Traffic 13 82 - 93; http://dx.doi.org/10.1111/j.1600-0854.2011.01294.x
- Fukuda M, Kanno E, Ishibashi K, Itoh T. Large scale screening for novel rab effectors reveals unexpected broad Rab binding specificity. Mol Cell Proteomics 2008; 7:1031 - 42; http://dx.doi.org/10.1074/mcp.M700569-MCP200; PMID: 18256213
- Kieken F, Sharma M, Jovic M, Giridharan SS, Naslavsky N, Caplan S, et al. Mechanism for the selective interaction of C-terminal Eps15 homology domain proteins with specific Asn-Pro-Phe-containing partners. J Biol Chem 2010; 285:8687 - 94; http://dx.doi.org/10.1074/jbc.M109.045666; PMID: 20106972
- Peränen J. Rab8 GTPase as a regulator of cell shape. Cytoskeleton (Hoboken) 2011; 68:527 - 39; http://dx.doi.org/10.1002/cm.20529; PMID: 21850707
- Arimura N, Menager C, Fukata Y, Kaibuchi K. Role of CRMP-2 in neuronal polarity. J Neurobiol 2004; 58:34 - 47; http://dx.doi.org/10.1002/neu.10269; PMID: 14598368
- Arimura N, Hattori A, Kimura T, Nakamuta S, Funahashi Y, Hirotsune S, et al. CRMP-2 directly binds to cytoplasmic dynein and interferes with its activity. J Neurochem 2009; 111:380 - 90; http://dx.doi.org/10.1111/j.1471-4159.2009.06317.x; PMID: 19659462
- Shi A, Chen CC, Banerjee R, Glodowski D, Audhya A, Rongo C, et al. EHBP-1 functions with RAB-10 during endocytic recycling in Caenorhabditis elegans. Mol Biol Cell 2010; 21:2930 - 43; http://dx.doi.org/10.1091/mbc.E10-02-0149; PMID: 20573983
- Chua CE, Lim YS, Tang BL. Rab35–a vesicular traffic-regulating small GTPase with actin modulating roles. FEBS Lett 2010; 584:1 - 6; http://dx.doi.org/10.1016/j.febslet.2009.11.051; PMID: 19931531
- Hsu C, Morohashi Y, Yoshimura S, Manrique-Hoyos N, Jung S, Lauterbach MA, et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol 2010; 189:223 - 32; http://dx.doi.org/10.1083/jcb.200911018; PMID: 20404108
- Egami Y, Fukuda M, Araki N. Rab35 regulates phagosome formation through recruitment of ACAP2 in macrophages during FcγR-mediated phagocytosis. J Cell Sci 2011; 124:3557 - 67; http://dx.doi.org/10.1242/jcs.083881; PMID: 22045739
- Kobayashi, H. & Fukuda, M. Rab35 regulates Arf6 activity through centaurin beta2/ACAP2 during neurite outgrowth. J Cell Sci, 2012: doi:jcs.098657 [pii]. 10.1242/jcs.098657.
- Kanno E, Ishibashi K, Kobayashi H, Matsui T, Ohbayashi N, Fukuda M. Comprehensive screening for novel rab-binding proteins by GST pull-down assay using 60 different mammalian Rabs. Traffic 2010; 11:491 - 507; http://dx.doi.org/10.1111/j.1600-0854.2010.01038.x; PMID: 20070612
- Donaldson JG, Honda A. Localization and function of Arf family GTPases. Biochem Soc Trans 2005; 33:639 - 42; http://dx.doi.org/10.1042/BST0330639; PMID: 16042562
- Caplan S, Naslavsky N, Hartnell LM, Lodge R, Polishchuk RS, Donaldson JG, et al. A tubular EHD1-containing compartment involved in the recycling of major histocompatibility complex class I molecules to the plasma membrane. EMBO J 2002; 21:2557 - 67; http://dx.doi.org/10.1093/emboj/21.11.2557; PMID: 12032069