Abstract
Embryonic stem (ES) cells have a pluripotent ability to differentiate into a variety of cell lineages in vitro. Using an embryoid body (EB) culture system, we developed a gut-like three-dimensional structure from mouse ES cells (the ES 3-D structure). Genetic studies implicate fibroblast growth factor 10 (FGF10)-FGF receptor 2b (FGFR2b) signaling as a critical regulator of lung bud morphogenesis in the embryonic foregut. The aim of the present study was to form a putative respiratory tract in the ES 3-D structure. By local application of FGF10 protein, we successfully demonstrated in vitro morphological formation of putative primitive respiratory tract-like processes, or buds, in the ES 3-D structure. Such organs that are differentiated from ES cells may provide new insights into tissue engineering and regenerative medicine.
Introduction
Signaling by fibroblast growth factor 10 (FGF10) and its receptor FGF receptor 2b (FGFR2b) is critical for bud morphogenesis in many developing structures.Citation1 During organogenesis, FGF10 is expressed by the mesoderm and subsequently diffuses to activate FGFR2b in adjacent endodermal- or ectodermal-derived cells and induce budding.Citation1-Citation4 In the earliest stages of lung development, FGF10 regulates branching morphogenesis via its receptors on the foregut, and disruption of FGF10-FGFR2b signaling is lethal at birth and results in defects in multiple organs, including the lungs.Citation5-Citation7
Recently, embryonic stem (ES) cells were shown to spontaneously give rise to a functional gut-like unit called the ES gut, which consists of a broad array of enteric derivatives from all three embryonic germ layers, including epithelial cells (endoderm), smooth muscle cells and interstitial cells of Cajal (mesoderm), and a small number of diffusely distributed enteric neurons (ectoderm).Citation8,Citation9 We successfully reproduced in vitro morphological development of mouse ES cell-derived three-dimensional structure similar to ES gut, which we designated the ES 3-D structure. Although nothing is known about the targets of FGF10-FGFR2b signaling in the ES 3-D structure, we hypothesized that primitive lung bud formation may be induced on the ES 3-D structure as a result of FGF10-FGFR2b signaling activation. In the present study, we demonstrate for the first time that local application of FGF10 induces differentiation of bud-like processes from the inner layer of ES 3-D structure. Our results open up a new vista of possibilities in the fields of tissue engineering and regenerative medicine.
Materials and Methods
Undifferentiated ES cells (EB3 cells) were maintained on gelatin-coated dishes without feeder cells in Dulbecco’s modified Eagle’s medium (Sigma) supplemented with 10% fetal bovine serum (Gibco/BRL), 0.1 mM 2-mercaptoethanol (Wako), 0.1 mM nonessential amino acids (Gibco/BRL), 1 mM sodium pyruvate (BioWhittaker Molecular Applications), and 1,000 U/ml leukemia inhibitory factor (LIF; Chemicon). The EB3 cells originated with Dr. Hitoshi Niwa, Center for Developmental Biology, Riken, Kobe. To induce embryoid body (EB) formation, dissociated ES cells were cultured in hanging drops as previously described, with minor modifications.Citation10 The cell density of one drop was 500 cells per 15 μl ES cell medium, without LIF. EBs were formed after 7 d in hanging drop culture. These EBs were transferred to outgrowth cultures on 100-mm gelatin-coated plastic dishes and allowed to attach. In each outgrowth culture, cell clusters underwent a dramatic transformation into cyst-like structures with a cavity containing fluid and solids. After observation of small cyst-like structures (on day 14), we planted heparin acrylic beads (Sigma), which were pre-soaked for 4 h in either control buffer (phosphate-buffered saline; PBS) or FGF10 (100 ng/μl, Sigma), near the cyst-like structures. Cells and organs were maintained in a humidified incubator at 37°C with 5% CO2 and images were acquired using a phase-contrast microscope (Nikon).
Results and Discussion
ES cells were cultured for 7 d in a hanging drop culture system and allowed to form aggregates called EBs, which were subsequently transferred to gelatin-coated dishes. After 5 to 7 d in culture, multiple clusters within each developing EB began to contract spontaneously with an irregular rhythm as the cells differentiated into beating cardiac muscle cells. Consistent with previous reports,Citation8-Citation11 each contracting cluster then underwent a dramatic transformation into a hemispherical dome-like 3-D structure ().
Figure 1. (A) A typical mouse ES cell-derived three-dimensional structure (ES 3-D structure) on day 16 of embryoid body (EB) outgrowth culture. (B) Representative example of ES 3-D structure planted control heparin beads (day 16). No morphological differences were observed among these conditions during our culturing protocol. (C) Representative example of ES 3-D structure treated with heparin beads soaked in FGF10 (day 16). Bud-like processes extending from the structural inner layer were observed. (D and E) Higher magnification of the bud-like processes within the white squares in (C). (lu) structural lumen; (be) heparin-coated bead. Scale bar indicates 100 μm. Arrows indicate the bud-like processes.
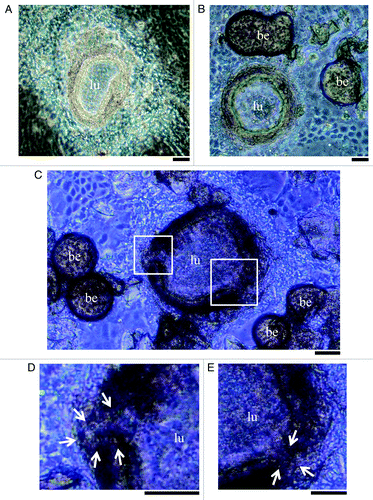
After observation of small cyst-like structures on day 14, we planted heparin-coated beads that were pre-soaked in either PBS or FGF10 near the cyst-like structures. As expected, the control heparin beads were not able to induce differentiation of bud-like organs in the ES 3-D structures (). In contrast, local application of FGF10 induced differentiation of bud-like processes from the intestinal inner layer in ES 3-D structures on day 16 (). These results suggest that the formation of bud-like processes may be induced by activation of FGF10 signaling in the ES 3-D structure.
Genetic data indicate that FGF10-FGFR2b signaling is required for bud formation in multiple developing structures during organogenesis.Citation1-Citation4 In the developing respiratory tract of the mouse, FGF10 is first detected at embryonic day 9.5 during primary bud formation.Citation7,Citation12,Citation13 The importance of FGF10 in lung development is demonstrated by the fact that FGF10 null mice die at birth due to numerous defects, one of which is the absence of lung buds.Citation14,Citation15 Using lung explant culture, it has been demonstrated that FGF10 acts as a chemo-attractant for the epithelium in lung buds in vitro. Furthermore, FGF10 expression studies suggest that FGF10 signaling plays an iterative role during lung branching morphogenesis in vivo, as FGF10 is expressed in a dynamic pattern at the tip of each forming bud.Citation5-Citation7,Citation12-Citation16
Our results provide the first evidence that FGF10 signaling may play a role in morphological formation of buds in the ES 3-D structure. We presume that these buds can be regarded as primitive respiratory tract-like processes, although we lack the biochemical evidence needed to definitively identify their molecular composition.
Recent studies have shown that induced pluripotent stem (iPS) cells can be generated from adult human dermal fibroblasts and other human somatic cells, and can be maintained on isogenic parental feeder cells.Citation17,Citation18 In combination with iPS cells, primitive lung bud formation within the 3-D structure represents a potential revolution in the fields of tissue engineering and regenerative medicine; for example, by opening the way for functional reconstruction after total laryngectomy.
| Abbreviations: | ||
| EB | = | embryoid body |
| EB3 cells | = | undifferentiated embryonic stem cells |
| ES | = | embryonic stem |
| FGF10 | = | fibroblast growth factor 10 |
| FGFR2b | = | fibroblast growth factor receptor 2b |
| iPS | = | induced pluripotent stem |
| LIF | = | leukemia inhibitory factor |
| PBS | = | phosphate-buffered saline |
Acknowledgments
The EB3 cells are the kind gift from Dr Miyako Takaki, Department of Physiology II, Nara Medical University, Kashihara, Nara. We thank Ms. Kanako Shoguchi (University of Occupational and Environmental Health, Kitakyushu, Japan) for technical assistance. This study was supported by a grant for Advanced Research from the University of Occupational and Environmental Health for Y.U., and by Grant-in-Aid for Scientific Research (B), No. 22390044 to Y.U. from Japan Society for the Promotion of Science.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Ohuchi H, Hori Y, Yamasaki M, Harada H, Sekine K, Kato S, et al. FGF10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochem Biophys Res Commun 2000; 277:643 - 9; http://dx.doi.org/10.1006/bbrc.2000.3721; PMID: 11062007
- Orr-Urtreger A, Bedford MT, Burakova T, Arman E, Zimmer Y, Yayon A, et al. Developmental localization of the splicing alternatives of fibroblast growth factor receptor-2 (FGFR2). Dev Biol 1993; 158:475 - 86; http://dx.doi.org/10.1006/dbio.1993.1205; PMID: 8393815
- Sekine K, Ohuchi H, Fujiwara M, Yamasaki M, Yoshizawa T, Sato T, et al. Fgf10 is essential for limb and lung formation. Nat Genet 1999; 21:138 - 41; http://dx.doi.org/10.1038/5096; PMID: 9916808
- De Moerlooze L, Spencer-Dene B, Revest JM, Hajihosseini M, Rosewell I, Dickson C. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development 2000; 127:483 - 92; PMID: 10631169
- Lü J, Izvolsky KI, Qian J, Cardoso WV. Identification of FGF10 targets in the embryonic lung epithelium during bud morphogenesis. J Biol Chem 2005; 280:4834 - 41; http://dx.doi.org/10.1074/jbc.M410714200; PMID: 15556938
- Bellusci S, Grindley J, Emoto H, Itoh N, Hogan BL. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development 1997; 124:4867 - 78; PMID: 9428423
- Kumar VH, Lakshminrusimha S, El Abiad MT, Chess PR, Ryan RM. Growth factors in lung development. Adv Clin Chem 2005; 40:261 - 316; http://dx.doi.org/10.1016/S0065-2423(05)40007-4; PMID: 16355925
- Yamada T, Yoshikawa M, Takaki M, Torihashi S, Kato Y, Nakajima Y, et al. In vitro functional gut-like organ formation from mouse embryonic stem cells. Stem Cells 2002; 20:41 - 9; http://dx.doi.org/10.1634/stemcells.20-1-41; PMID: 11796921
- Ishikawa T, Nakayama S, Nakagawa T, Horiguchi K, Misawa H, Kadowaki M, et al. Characterization of in vitro gutlike organ formed from mouse embryonic stem cells. Am J Physiol Cell Physiol 2004; 286:C1344 - 52; http://dx.doi.org/10.1152/ajpcell.00392.2003; PMID: 14960414
- Takaki M, Nakayama S, Misawa H, Nakagawa T, Kuniyasu H. In vitro formation of enteric neural network structure in a gut-like organ differentiated from mouse embryonic stem cells. Stem Cells 2006; 24:1414 - 22; http://dx.doi.org/10.1634/stemcells.2005-0394; PMID: 16527901
- Takaki M, Misawa H, Matsuyoshi H, Kawahara I, Goto K, Zhang GX, et al. In vitro enhanced differentiation of neural networks in ES gut-like organ from mouse ES cells by a 5-HT4-receptor activation. Biochem Biophys Res Commun 2011; 406:529 - 33; http://dx.doi.org/10.1016/j.bbrc.2011.02.072; PMID: 21333625
- Hyatt BA, Shangguan X, Shannon JM. FGF-10 induces SP-C and Bmp4 and regulates proximal-distal patterning in embryonic tracheal epithelium. Am J Physiol Lung Cell Mol Physiol 2004; 287:L1116 - 26; http://dx.doi.org/10.1152/ajplung.00033.2004; PMID: 15531758
- Hirashima T, Iwasa Y, Morishita Y. Mechanisms for split localization of Fgf10 expression in early lung development. Dev Dyn 2009; 238:2813 - 22; http://dx.doi.org/10.1002/dvdy.22108; PMID: 19842186
- Min H, Danilenko DM, Scully SA, Bolon B, Ring BD, Tarpley JE, et al. Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev 1998; 12:3156 - 61; http://dx.doi.org/10.1101/gad.12.20.3156; PMID: 9784490
- Nyeng P, Norgaard GA, Kobberup S, Jensen J. FGF10 maintains distal lung bud epithelium and excessive signaling leads to progenitor state arrest, distalization, and goblet cell metaplasia. BMC Dev Biol 2008; 8:2; http://dx.doi.org/10.1186/1471-213X-8-2; PMID: 18186922
- Ohtsuka N, Urase K, Momoi T, Nogawa H. Induction of bud formation of embryonic mouse tracheal epithelium by fibroblast growth factor plus transferrin in mesenchyme-free culture. Dev Dyn 2001; 222:263 - 72; http://dx.doi.org/10.1002/dvdy.1206; PMID: 11668603
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007; 131:861 - 72; http://dx.doi.org/10.1016/j.cell.2007.11.019; PMID: 18035408
- Takahashi K, Narita M, Yokura M, Ichisaka T, Yamanaka S. Human induced pluripotent stem cells on autologous feeders. PLoS One 2009; 4:e8067; http://dx.doi.org/10.1371/journal.pone.0008067; PMID: 19956543