Abstract
Understanding how planar cell polarity (PCP) is established, maintained, and coordinated in migrating cell populations is an important area of research with implications for both embryonic morphogenesis and tumor cell invasion. We recently reported that the PCP protein Vang-like 2 (VANGL2) regulates the endocytosis and cell surface level of membrane type-1 matrix metalloproteinase (MMP14 or MT1-MMP). Here, we further discuss these findings in terms of extracellular matrix (ECM) remodeling, cell migration, and zebrafish gastrulation. We also demonstrate that VANGL2 function impacts the focal degradation of ECM by human cancer cells including the formation or stability of invadopodia. Together, our findings implicate MMP14 as a downstream effector of VANGL2 signaling and suggest a model whereby the regulation of pericellular proteolysis is a fundamental aspect of PCP in migrating cells.
During zebrafish gastrulation, PCP is defined as the elongation and mediolateral alignment of cells as they engage in polarized behaviors including collective or group migration.Citation1-Citation4 Over a decade ago it was recognized that homologs of proteins regulating PCP in cuticular structures of Drosophila melanogaster also control PCP in gastrula cells.Citation2,Citation5,Citation6 Zebrafish gastrulation mutant embryos such as trilobite/vangl2 exhibit a PCP phenotype characterized by shortened and broadened embryonic body axes.Citation2,Citation7,Citation8 It is generally thought that vertebrate PCP signaling regulates the formation, polarization, and/or stabilization of actin-rich membrane protrusions.Citation9 This concept is largely based on data from the fly wing epithelium demonstrating that PCP proteins restrict the formation and localization actin-rich structures.Citation10 Indeed, Rho family small GTPases are known regulators of the actin cytoskeleton and influence gastrulation cell movements in the Xenopus laevis embryo.Citation11-Citation13 Disruption of membrane protrusive activity in the zebrafish gastrula is thought to underlie the PCP defect in trilobite/vangl2 mutant embryos.Citation2 However, in migrating cell populations the establishment of PCP must be coordinated with other proteins/pathways regulating motility including those affecting ECM remodeling and cell-matrix adhesion.Citation14 Therefore, identification of additional proteins regulating gastrulation cell movements and determination of how they interact with PCP signaling is crucial.
Previously our lab demonstrated that Mmp14 is required for PCP and exhibits a strong genetic interaction with knypek/glypican4,Citation15 a Wnt co-receptor necessary for proper gastrulation cell movements.Citation4 Subsequently we showed that a fibronectin- and laminin-containing ECM network develops coincidently with the timing of PCP establishment.Citation16 By late gastrulation stages fibronectin and laminin form two layers; one between the ectoderm and mesoderm germ layers and a second localizing beneath (and surrounding) deep mesodermal and endodermal cells.Citation16 Notably, utilizing a cancer cell culture model we further demonstrated that human VANGL2 regulates cell surface MMP14 expression, MMP2 activation, and invasion through an ECM barrier.Citation17 Taken together, our previous data suggested a mechanistic connection between the establishment of PCP in migrating cells and matrix metalloproteinase-dependent ECM remodeling.
In our recent work, we hypothesized that the transmembrane PCP protein VANGL2 directly regulates cell surface levels of MMP14 by controlling its trafficking to or from the plasma membrane.Citation18 To test this possibility we transfected human fibrosarcoma HT-1080 cells with either VANGL2 or control siRNAs and performed various endocytosis and recycling assays. These cells are frequently used to address mechanisms of MMP14 trafficking and localization to specific vesicular compartments.Citation19,Citation20 Utilizing a biochemical assay based on biotin labeling of cell surface proteins followed by shifting the temperature to 37°C, it became clear that VANGL2 knockdown cells have a defect in MMP14 endocytosis. Significantly, loss of VANGL2 function did not globally disrupt trafficking as indicated by endocytosis of transferrin.
In the embryo VANGL2 homologs are thought to function at the plasma membrane but their expression has been reported in both membrane and vesicular compartments,Citation21,Citation22 a finding that we also observed in HT-1080 cells.Citation18 Thus it was unclear whether VANGL2 signaling acted at the cell surface to influence MMP14 internalization. Previous data showed that endocytosis of MMP14 could be regulated downstream of focal adhesion kinase (FAK) phosphorylation at Y-397.Citation23 This suggests that MMP14 trafficking might be coordinated with integrin function and the formation of cell-matrix adhesions. Indeed, we demonstrated that VANGL2-dependent effects on cell surface MMP14 required Y-397 phosphorylation of FAK.Citation18 These results suggest that in HT-1080 cells VANGL2 regulates MMP14 endocytosis at the level of the plasma membrane.
Based on our cell culture data we further hypothesized that zebrafish trilobite/vangl2 mutant embryos have increased matrix metalloproteinase activity. By extracting total embryo protein under conditions that maintain enzymatic activity, we were able to perform protease assays using fluorogenic gelatin and collagen IV substrates. We found that trilobite/vangl2 mutant embryos have significantly more protease activity than wild-type controls and that this activity could be suppressed using broad-spectrum or Mmp14-specific inhibitors.Citation18 Moreover, by knocking down Mmp14 in Vangl2 loss of function embryos we were able to suppress the gastrulation cell movement defect indicating that Mmp14 acts downstream of Vangl2. We next determined whether loss of Vangl2 function during gastrulation affected formation of an ECM network. By immunolabeling for fibronectin and performing confocal microscopic imaging we showed that trilobite/vangl2 mutant embryos have decreased ECM.Citation18 These biochemical and molecular genetic data indicate that Vangl2-dependent regulation of Mmp14 activity is required for fibronectin remodeling in the zebrafish gastrula embryo. It is also likely that Mmp14 acts on additional ECM and non-ECM substrates to influence PCP during gastrulation.
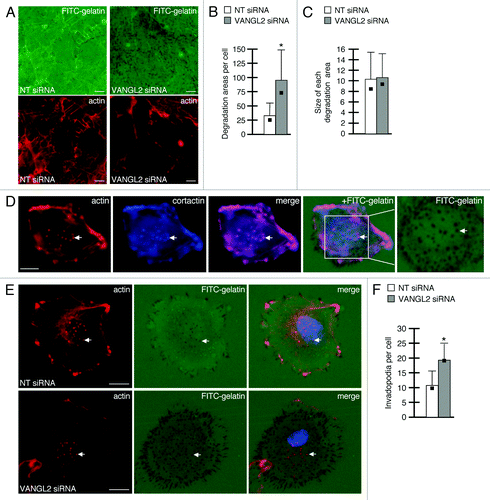
In the fly wing epithelium, Van Gogh restricts or localizes the activity of other PCP proteins to specific polarized cellular domains.Citation24 We therefore wondered whether human VANGL2 regulates cell surface proteolytic activity and focal matrix degradation at polarized plasma membrane structures including protrusions and invadopodia. First, we incubated HT-1080 cells on fluorescent gelatin for 20 h and quantified the total degradation area in relation to cell number. Here, the focal ECM degradation areas detected resembled footprints or tracks created by protease activity that is associated with membrane protrusions (). Our data show that VANGL2 siRNA transfected cells have significantly more degradation areas per cell than controls () though the average size is not increased in VANGL2 knockdown cells (). In contrast to the degradation areas produced by membrane protrusions, invadopodia are dot-like F-actin-rich structures that are formed at certain cell-matrix contact sites and exhibit increased MMP14 activity and ECM degradation.Citation25 To visualize invadopodia, we incubated HT-1080 cells on fluorescently labeled gelatin for 5 h prior to fixation and imaging. We identified actin-positive punctae that both co-labeled with cortactin and overlapped with foci of matrix degradation (). These structures are thus considered invadopodiaCitation25 and were quantified in VANGL2 and control non-targeting siRNA transfected cells. Our results indicate that VANGL2 knockdown cells have more invadopodia than controls (). Notably, the size of invadopodium and their associated matrix degradation spots appeared larger in VANGL2 knockdown cells than controls (). However, because HT-1080 cells are highly motile on 2D ECM substrates,Citation17 we were unable to quantify the focal degradation spots produced specifically by the invadopodia of individual cells. Together, our results support the notion that increased cell surface proteolytic activity in VANGL2 knockdown cells increases total focal matrix degradation and affects the formation or stability of invadopodia. Our data are consistent with observations that loss of MMP14 function disrupts both invadopodia formation and proteolytic activity.Citation26,Citation27
Figure 1. VANGL2 regulates the focal degradation of ECM by HT-1080 cancer cells. (A) Total degradation areas observed after 20 h incubation on FITC-labeled gelatin (actin-labeled images also shown). Quantification of (B) average number of degradation areas formed (normalized to total cell number) and (C) average size of each degradation area with standard deviations and medians (black boxes). (D) Invadopodia formed after 5 h incubation on FITC-labeled gelatin as visualized by phalloidin (to label actin), cortactin, and matrix degradation (arrows). Area within the white box is magnified in adjacent panel. (E) Invadopodia formation in control non-targeting (NT) and VANGL2 siRNA transfected cells. Arrows denote matrix degradation spots co-localizing with actin foci (only one spot is highlighted per cell). (F) Quantification of the average number of invadopodia per cell with standard deviations and medians (black boxes). (B and F) Asterisks indicate p-value less than 0.01. Scale bars = 10 μm.
In summary, we have demonstrated that the PCP protein VANGL2 regulates MMP14 endocytosis and cell surface activity and that this membrane-tethered protease functions downstream of zebrafish Vangl2 to influence both ECM remodeling and gastrulation cell movements.Citation18 Together with other work,Citation28-Citation30 these data suggest that the regulation of vesicular trafficking events may be a broadly applicable mechanism underlying the establishment of PCP in diverse cell types. We have now shown that human VANGL2 also impacts the focal degradation of ECM and the formation or stability of invadopodia. It will now be important to determine how zebrafish Vangl2 function influences polarized cell behaviors underlying collective or group migration. During gastrulation, migrating cells interact with ECM proteins but do not plow through or invade an ECM barrier.Citation16 In this context, we suggest that tight regulation of cell surface MMP14 activity at cell-matrix focal adhesions is required to restrict membrane protrusive activity to specific cellular domains.
Materials and Methods
Control and VANGL2 knockdown cells were generated as described.Citation17 The QCMTM gelatin invadopodia assays were performed and quantified according to manufacturer instructions (Millipore, ECM670). In each experiment 20,000 HT-1080 cells were plated per well of an 8-well chamber slide. The total number of degradation spots formed after 20 h incubation (including both invadopodia and other protrusive membrane structures) was quantified from three independent experiments (12 images per experiment = 36 fields of view analyzed, > 500 cells per condition). The number of invadopodia formed after 5 h was also quantified from three independent experiments (20 cells per experiment = 60 total cells per condition). Only cells with at least one invadopodium were analyzed. Cortactin antibody labeling (Millipore clone 4F11; 1:500 dilution) was detected using a mouse Cy5 secondary antibody (Jackson ImmunoResearch; 1:400 dilution). Statistical significance was calculated utilizing the unpaired Student’s ttest.
| Abbreviations: | ||
| ECM | = | extracellular matrix |
| FAK | = | focal adhesion kinase |
| MMP14 | = | membrane type-1 matrix metalloproteinase |
| PCP | = | planar cell polarity |
| VANGL2 | = | Vang-like 2 |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by grants to J.R.J. from the American Cancer Society (RSG 0928101) and National Science Foundation (IOS 0950849).
References
- Jessen JR, Solnica-Krezel L. Morphogenetic cell movements shaping the zebrafish gastrula. In: Mlodzik M, editor. Planar cell polarization during development. San Diego: Elsevier Press; 2005. p. 131-165.
- Jessen JR, Topczewski J, Bingham S, Sepich DS, Marlow F, Chandrasekhar A, et al. Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nat Cell Biol 2002; 4:610 - 5; PMID: 12105418
- Marlow F, Topczewski J, Sepich D, Solnica-Krezel L. Zebrafish Rho kinase 2 acts downstream of Wnt11 to mediate cell polarity and effective convergence and extension movements. Curr Biol 2002; 12:876 - 84; http://dx.doi.org/10.1016/S0960-9822(02)00864-3; PMID: 12062050
- Topczewski J, Sepich DS, Myers DC, Walker C, Amores A, Lele Z, et al. The zebrafish glypican knypek controls cell polarity during gastrulation movements of convergent extension. Dev Cell 2001; 1:251 - 64; http://dx.doi.org/10.1016/S1534-5807(01)00005-3; PMID: 11702784
- Djiane A, Riou J, Umbhauer M, Boucaut J, Shi D. Role of frizzled 7 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development 2000; 127:3091 - 100; PMID: 10862746
- Wallingford JB, Rowning BA, Vogeli KM, Rothbächer U, Fraser SE, Harland RM. Dishevelled controls cell polarity during Xenopus gastrulation. Nature 2000; 405:81 - 5; http://dx.doi.org/10.1038/35011077; PMID: 10811222
- Sepich DS, Myers DC, Short R, Topczewski J, Marlow F, Solnica-Krezel L. Role of the zebrafish trilobite locus in gastrulation movements of convergence and extension. Genesis 2000; 27:159 - 73; http://dx.doi.org/10.1002/1526-968X(200008)27:4<159::AID-GENE50>3.0.CO;2-T; PMID: 10992326
- Solnica-Krezel L, Stemple DL, Mountcastle-Shah E, Rangini Z, Neuhauss SC, Malicki J, et al. Mutations affecting cell fates and cellular rearrangements during gastrulation in zebrafish. Development 1996; 123:67 - 80; PMID: 9007230
- Roszko I, Sawada A, Solnica-Krezel L. Regulation of convergence and extension movements during vertebrate gastrulation by the Wnt/PCP pathway. Semin Cell Dev Biol 2009; 20:986 - 97; http://dx.doi.org/10.1016/j.semcdb.2009.09.004; PMID: 19761865
- Adler PN. Planar signaling and morphogenesis in Drosophila. Dev Cell 2002; 2:525 - 35; http://dx.doi.org/10.1016/S1534-5807(02)00176-4; PMID: 12015961
- Habas R, Dawid IB, He X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev 2003; 17:295 - 309; http://dx.doi.org/10.1101/gad.1022203; PMID: 12533515
- Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell 2001; 107:843 - 54; http://dx.doi.org/10.1016/S0092-8674(01)00614-6; PMID: 11779461
- Tahinci E, Symes K. Distinct functions of Rho and Rac are required for convergent extension during Xenopus gastrulation. Dev Biol 2003; 259:318 - 35; http://dx.doi.org/10.1016/S0012-1606(03)00206-9; PMID: 12871704
- Jessen JR. Noncanonical Wnt signaling in tumor progression and metastasis. Zebrafish 2009; 6:21 - 8; http://dx.doi.org/10.1089/zeb.2008.0571; PMID: 19292672
- Coyle RC, Latimer A, Jessen JR. Membrane-type 1 matrix metalloproteinase regulates cell migration during zebrafish gastrulation: evidence for an interaction with non-canonical Wnt signaling. Exp Cell Res 2008; 314:2150 - 62; http://dx.doi.org/10.1016/j.yexcr.2008.03.010; PMID: 18423448
- Latimer A, Jessen JR. Extracellular matrix assembly and organization during zebrafish gastrulation. Matrix Biol 2010; 29:89 - 96; http://dx.doi.org/10.1016/j.matbio.2009.10.002; PMID: 19840849
- Cantrell VA, Jessen JR. The planar cell polarity protein Van Gogh-Like 2 regulates tumor cell migration and matrix metalloproteinase-dependent invasion. Cancer Lett 2010; 287:54 - 61; http://dx.doi.org/10.1016/j.canlet.2009.05.041; PMID: 19577357
- Williams BB, Cantrell VA, Mundell NA, Bennett AC, Quick RE, Jessen JR. VANGL2 regulates membrane trafficking of MMP14 to control cell polarity and migration. J Cell Sci 2012; in press http://dx.doi.org/10.1242/jcs.097964; PMID: 22357946
- Jiang A, Lehti K, Wang X, Weiss SJ, Keski-Oja J, Pei D. Regulation of membrane-type matrix metalloproteinase 1 activity by dynamin-mediated endocytosis. Proc Natl Acad Sci U S A 2001; 98:13693 - 8; http://dx.doi.org/10.1073/pnas.241293698; PMID: 11698655
- Remacle A, Murphy G, Roghi C. Membrane type I-matrix metalloproteinase (MT1-MMP) is internalised by two different pathways and is recycled to the cell surface. J Cell Sci 2003; 116:3905 - 16; http://dx.doi.org/10.1242/jcs.00710; PMID: 12915589
- Cha SW, Tadjuidje E, Wylie C, Heasman J. The roles of maternal Vangl2 and aPKC in Xenopus oocyte and embryo patterning. Development 2011; 138:3989 - 4000; http://dx.doi.org/10.1242/dev.068866; PMID: 21813572
- Ciruna B, Jenny A, Lee D, Mlodzik M, Schier AF. Planar cell polarity signalling couples cell division and morphogenesis during neurulation. Nature 2006; 439:220 - 4; http://dx.doi.org/10.1038/nature04375; PMID: 16407953
- Wu X, Gan B, Yoo Y, Guan JL. FAK-mediated src phosphorylation of endophilin A2 inhibits endocytosis of MT1-MMP and promotes ECM degradation. Dev Cell 2005; 9:185 - 96; http://dx.doi.org/10.1016/j.devcel.2005.06.006; PMID: 16054026
- Strutt DI. The asymmetric subcellular localisation of components of the planar polarity pathway. Semin Cell Dev Biol 2002; 13:225 - 31; http://dx.doi.org/10.1016/S1084-9521(02)00041-1; PMID: 12137731
- Linder S, Wiesner C, Himmel M. Degrading devices: invadosomes in proteolytic cell invasion. Annu Rev Cell Dev Biol 2011; 27:185 - 211; http://dx.doi.org/10.1146/annurev-cellbio-092910-154216; PMID: 21801014
- Clark ES, Whigham AS, Yarbrough WG, Weaver AM. Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer Res 2007; 67:4227 - 35; http://dx.doi.org/10.1158/0008-5472.CAN-06-3928; PMID: 17483334
- Steffen A, Le Dez G, Poincloux R, Recchi C, Nassoy P, Rottner K, et al. MT1-MMP-dependent invasion is regulated by TI-VAMP/VAMP7. Curr Biol 2008; 18:926 - 31; http://dx.doi.org/10.1016/j.cub.2008.05.044; PMID: 18571410
- Classen AK, Anderson KI, Marois E, Eaton S. Hexagonal packing of Drosophila wing epithelial cells by the planar cell polarity pathway. Dev Cell 2005; 9:805 - 17; http://dx.doi.org/10.1016/j.devcel.2005.10.016; PMID: 16326392
- Gray RS, Abitua PB, Wlodarczyk BJ, Szabo-Rogers HL, Blanchard O, Lee I, et al. The planar cell polarity effector Fuz is essential for targeted membrane trafficking, ciliogenesis and mouse embryonic development. Nat Cell Biol 2009; 11:1225 - 32; http://dx.doi.org/10.1038/ncb1966; PMID: 19767740
- Ulrich F, Krieg M, Schötz EM, Link V, Castanon I, Schnabel V, et al. Wnt11 functions in gastrulation by controlling cell cohesion through Rab5c and E-cadherin. Dev Cell 2005; 9:555 - 64; http://dx.doi.org/10.1016/j.devcel.2005.08.011; PMID: 16198297