Abstract
Lipid rafts are highly dynamic membrane subdomains enriched in specific protein and lipid components that create specialized ‘organizing’ platforms essential for an array of important cellular functions. The role of lipid rafts in membrane trafficking involves the constant remodelling of the plasma membrane through membrane uptake and balanced exocytosis of intracellular membranes. Our lab has identified the first motor protein, myosin 1c (Myo1c) involved in driving the recycling of lipid-raft enriched membranes from the perinuclear recycling compartment to the cell surface. This newly discovered role for Myo1c in lipid raft exocytosis is crucial for cell spreading, migration and pathogen entry; key cellular processes that require cell surface expansion and plasticity. Here we present a model suggesting Myo1c’s possible molecular functions in lipid raft recycling and discuss its wider implications for important cellular functions.
The plasma membrane in eukaryotic cells is in a constant state of flux undergoing dynamic remodeling by endocytosis (membrane uptake) and membrane reinsertion (recycling/exocytosis), which controls its plasticity by regulating the composition of the cell surface. Certain membrane lipids (e.g., cholesterol, sphingolipids) and proteins assemble into specialized microdomains, termed lipid rafts that compartmentalize crucial cellular processes such as membrane trafficking and signaling.Citation1,Citation2 Endocytosis and recycling of lipid raft microdomains are specialized actin-dependent pathways, for which the molecular determinants remain elusive. Our lab however has recently established that the actin-based motor protein myosin1c (Myo1c) promotes endocytic recycling by controlling the generation of lipid raft-enriched tubular recycling carriers extending from the recycling compartment.Citation3 We demonstrated that Myo1c is a lipid raft-associated motor protein that specifically controls recycling of lipid raft-associated cargo to the plasma membrane. Abolishing Myo1c function by RNA interference or overexpressing a dominant-negative mutant induced a collapse of raft-enriched recycling tubules and lipid rafts accumulated in the recycling compartment causing a dramatic loss of raft markers from the cell surface. Conversely, overexpression of exogenous Myo1c increased lipid raft levels at the cell surface. Myo1c selectively regulated lipid raft exocytosis to the plasma membrane, but it was dispensable for recycling of cargo, such as the transferrin receptor, along separate parallel pathways. The dramatic defect in lipid raft recycling following Myo1c knockdown had a severe impact on cell spreading, cell migration and cholesterol-dependent Salmonella entry, demonstrating that Myo1c-mediated raft delivery to the cell surface plays a pivotal role in processes which require the correct targeting of signaling molecules and extra membrane fundamental for plasma membrane expansion and remodeling.Citation3
Molecular Roles of Myo1c Function in Lipid Raft Exocytosis
We observed that Myo1c is present at the plasma membrane and also on dynamic raft-enriched tubular carriers emanating from the perinuclear recycling compartment.Citation3 This dual localization opens the possibility that Myo1c performs one or more functions during lipid raft exocytosis ():
Figure 1. Model outlining the possible molecular roles of Myo1c in lipid raft recycling. (A) Myo1c could mediate cargo sorting at the endocytic recycling compartment (ERC), a process that is crucial for the correct recycling of proteins by distinct pathways. By linking lipid raft membranes and cargo proteins associated with these microdomains to adjacent actin filaments, Myo1c could cluster molecular cargo for subsequent transport to the cell surface. (B) Myo1c might initiate the generation of recycling tubules at the ERC by membrane deformation. By anchoring the membrane to surrounding actin filaments Myo1c motor activity could generate a force to deform the membrane for nascent tubular carriers. Through its ATP-driven powerstroke Myo1c could create the tension that would actively pull on the ERC membrane. Thereby Myo1c might prime the formation of tubule precursers, which may then be elongated toward the plasma membrane by microtubule-associated kinesin motors. (C) Myo1c could also be involved in the final stages of exocytosis, where it might propell cargo transport through the dense cortical actin filament network. It is also plausible that Myo1c together with its binding partner RalA and the exocyst complex mediates the docking and fusion of lipid raft enriched recycling carriers with the plasma membrane. These proposed activities are not mutually exclusive and it is possible that each might contribute to Myo1c function in lipid raft exocytosis.
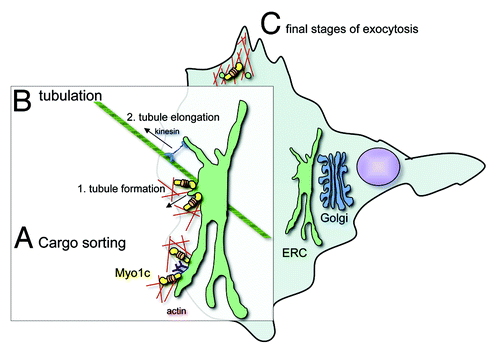
A) First of all, Myo1c could be involved in cargo sorting at the endocytic recycling compartment (ERC) (see ). At least two distinct pathways recycle cargo from a perinuclear ERC back to the plasma membrane; one of which transports lipid raft-associated cargo, while a separate pathway recycles cargoes internalized via clathrin-dependent endocytosis.Citation4 The sorting of distinct cargoes at the perinuclear recycling compartment is key to the recycling process and this may be facilitated by the Myo1c motor protein, which could link raft microdomains to the actin cytoskeleton primed for cargo transport along actin filaments.
B) In addition, Myo1c could promote the generation of tubular carriers in the perinuclear region by anchoring and pulling specific membranes along actin filaments to form tubules extending from the lipid raft-enriched recycling compartment (). Class I myosins are ‘single-headed’ motors (contain a single motor domain) that have been proposed to act as molecular force sensors, which upon increase of their cellular cargo load remain attached to actin filaments for longer periods, enabling them to generate and maintain tension for extended periods of time.Citation5 These mechanochemical properties support a role for Myo1c in controlling membrane tension and in promoting membrane deformation underlying the initial stages of tubule formation. Most likely these tubule precursors are then elongated and transported toward the plasma membrane by microtubule-associated motor proteins such as kinesins, which have previously been observed to accumulate at the tip of tubular membrane carriers and to actively drive tubule extension.Citation6,Citation7
Intriguingly, another myosin class I member, Myo1b, has recently been shown to promote tubule formation at the TGN.Citation8 Myo1b was found to initiate the tubulation of post-Golgi carriers by regulating actin assembly and remodeling TGN membranes.
C) Like its function in transport of GLUT4-positive vesicles in adipocytes,Citation9,Citation10 Myo1c could also mediate the final steps of exocytosis through the cortical actin network beneath the plasma membrane, where it might promote, together with its binding partner the GTPase RalA and the exocyst complex, the delivery and fusion of lipid raft-containing membranes with the plasma membrane (). A role for Myo1c in the final stages of exocytosis is supported by the observation that Myo1c is required during the regulated exocytosis of cortical granules (CGs) in Xenopus laevis oocytes.Citation11 To block polyspermy egg fertilization induces an increase of intracellular Ca2+, which triggers the stimulated exocytosis of CGs, a process that is driven by the compression of the actin filament coat surrounding the CGs. Myo1c is recruited to secretory CGs and disruption of its function resulted in suppressed exocytosis.Citation11 In this process during the final stages of CG secretion Myo1c is believed to link and regulate actin filament polymerisation on the surface of CG and so mediate force production.
Cellular Processes Dependent on Myo1c-Mediated Raft Exocytosis
The severe defect in lipid raft targeting to the cell surface in Myo1c depleted cells has a profound impact on cellular processes that require the dynamic remodelling and expansion of the plasma membrane. This defect was found to impair leading edge protrusion, underlying cell spreading, migration and cholesterol-dependent Salmonella invasion. In summary, these novel roles for Myo1c suggest that it may act as a general regulator of stimulated exocytosis by utilizing its ability to link lipid raft microdomains, actin filaments and the RalA-mediated exocytic machinery for cargo delivery. Myo1c does indeed facilitate the transport of diverse raft-associated cargoes including GLUT4,Citation12 aquaporin-213 and Neph1Citation14 to the cell surface. Moreover, RalA and the exocyst complex are also involved in the translocation of vesicles containing the GLUT4 transporter and aquaporin-2,Citation12,Citation13,Citation15 suggesting that Myo1c, RalA and the exocyst complex are part of the core machinery required for raft exocytosis. What are the lipid raft-associated cargoes of Myo1c that might regulate cell spreading, migration and bacterial invasion? The central cytoskeletal regulators Rac1 and Cdc42 localize to raft microdomains, which are known to modulate small GTPase targeting and activation.Citation16,Citation17 Importantly, defective lipid raft trafficking was observed to mislocalize Rac1, which blocked cell spreading, migration and Salmonella-induced macropinocytosis.Citation18,Citation19 In addition, Myo1c may supply membranes to influence cell plasticity, as there is recent evidence that the exocytosis of lipid rafts not only delivers key protein components to the plasma membrane, but also provides the extra membrane required for cell surface expansion.Citation20 This would be consistent with Myo1c-dependent formation of raft-enriched membrane-tubules, which emanate from a previously defined ‘membrane-storage’ compartment.Citation20 Thus the diverse lipid and protein composition of raft microdomains is reflected in the array of pathways in which they participate, indicating a pivotal role for Myo1c in a range of cellular processes.
References
- Simons K, Gerl MJ. Revitalizing membrane rafts: new tools and insights. Nat Rev Mol Cell Biol 2010; 11:688 - 99; http://dx.doi.org/10.1038/nrm2977; PMID: 20861879
- Simons K, Gruenberg J. Jamming the endosomal system: lipid rafts and lysosomal storage diseases. Trends Cell Biol 2000; 10:459 - 62; http://dx.doi.org/10.1016/S0962-8924(00)01847-X; PMID: 11050411
- Brandstaetter H, Kendrick-Jones J, Buss F. Myo1c regulates lipid raft recycling to control cell spreading, migration and Salmonella invasion. J Cell Sci 2012; 125:1991 - 2003; http://dx.doi.org/10.1242/jcs.097212; PMID: 22328521
- Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol 2009; 10:597 - 608; http://dx.doi.org/10.1038/nrm2755; PMID: 19696797
- Laakso JM, Lewis JH, Shuman H, Ostap EM. Myosin I can act as a molecular force sensor. Science 2008; 321:133 - 6; http://dx.doi.org/10.1126/science.1159419; PMID: 18599791
- Leduc C, Campàs O, Zeldovich KB, Roux A, Jolimaitre P, Bourel-Bonnet L, et al. Cooperative extraction of membrane nanotubes by molecular motors. Proc Natl Acad Sci U S A 2004; 101:17096 - 101; http://dx.doi.org/10.1073/pnas.0406598101; PMID: 15569933
- Roux A, Cappello G, Cartaud J, Prost J, Goud B, Bassereau P. A minimal system allowing tubulation with molecular motors pulling on giant liposomes. Proc Natl Acad Sci U S A 2002; 99:5394 - 9; http://dx.doi.org/10.1073/pnas.082107299; PMID: 11959994
- Almeida CG, Yamada A, Tenza D, Louvard D, Raposo G, Coudrier E. Myosin 1b promotes the formation of post-Golgi carriers by regulating actin assembly and membrane remodelling at the trans-Golgi network. Nat Cell Biol 2011; 13:779 - 89; http://dx.doi.org/10.1038/ncb2262; PMID: 21666684
- Bose A, Guilherme A, Robida SI, Nicoloro SM, Zhou QL, Jiang ZY, et al. Glucose transporter recycling in response to insulin is facilitated by myosin Myo1c. Nature 2002; 420:821 - 4; http://dx.doi.org/10.1038/nature01246; PMID: 12490950
- Bose A, Robida S, Furcinitti PS, Chawla A, Fogarty K, Corvera S, et al. Unconventional myosin Myo1c promotes membrane fusion in a regulated exocytic pathway. Mol Cell Biol 2004; 24:5447 - 58; http://dx.doi.org/10.1128/MCB.24.12.5447-5458.2004; PMID: 15169906
- Sokac AM, Schietroma C, Gundersen CB, Bement WM. Myosin-1c couples assembling actin to membranes to drive compensatory endocytosis. Dev Cell 2006; 11:629 - 40; http://dx.doi.org/10.1016/j.devcel.2006.09.002; PMID: 17084356
- Chen XW, Leto D, Chiang SH, Wang Q, Saltiel AR. Activation of RalA is required for insulin-stimulated Glut4 trafficking to the plasma membrane via the exocyst and the motor protein Myo1c. Dev Cell 2007; 13:391 - 404; http://dx.doi.org/10.1016/j.devcel.2007.07.007; PMID: 17765682
- Barile M, Pisitkun T, Yu MJ, Chou CL, Verbalis MJ, Shen RF, et al. Large scale protein identification in intracellular aquaporin-2 vesicles from renal inner medullary collecting duct. Mol Cell Proteomics 2005; 4:1095 - 106; http://dx.doi.org/10.1074/mcp.M500049-MCP200; PMID: 15905145
- Arif E, Wagner MC, Johnstone DB, Wong HN, George B, Pruthi PA, et al. Motor protein Myo1c is a podocyte protein that facilitates the transport of slit diaphragm protein Neph1 to the podocyte membrane. Mol Cell Biol 2011; 31:2134 - 50; http://dx.doi.org/10.1128/MCB.05051-11; PMID: 21402783
- Yu MJ, Pisitkun T, Wang G, Aranda JF, Gonzales PA, Tchapyjnikov D, et al. Large-scale quantitative LC-MS/MS analysis of detergent-resistant membrane proteins from rat renal collecting duct. Am J Physiol Cell Physiol 2008; 295:C661 - 78; http://dx.doi.org/10.1152/ajpcell.90650.2007; PMID: 18596208
- del Pozo MA, Alderson NB, Kiosses WB, Chiang HH, Anderson RG, Schwartz MA. Integrins regulate Rac targeting by internalization of membrane domains. Science 2004; 303:839 - 42; http://dx.doi.org/10.1126/science.1092571; PMID: 14764880
- Golub T, Pico C. Spatial control of actin-based motility through plasmalemmal PtdIns(4,5)P2-rich raft assemblies. Biochem Soc Symp 2005; 72:119 - 27; PMID: 15649136
- Balasubramanian N, Scott DW, Castle JD, Casanova JE, Schwartz MA. Arf6 and microtubules in adhesion-dependent trafficking of lipid rafts. Nat Cell Biol 2007; 9:1381 - 91; http://dx.doi.org/10.1038/ncb1657; PMID: 18026091
- Misselwitz B, Dilling S, Vonaesch P, Sacher R, Snijder B, Schlumberger M, et al. RNAi screen of Salmonella invasion shows role of COPI in membrane targeting of cholesterol and Cdc42. Mol Syst Biol 2011; 7:474; http://dx.doi.org/10.1038/msb.2011.7; PMID: 21407211
- Gauthier NC, Rossier OM, Mathur A, Hone JC, Sheetz MP. Plasma membrane area increases with spread area by exocytosis of a GPI-anchored protein compartment. Mol Biol Cell 2009; 20:3261 - 72; http://dx.doi.org/10.1091/mbc.E09-01-0071; PMID: 19458190