Abstract
Deregulated apoptosis is a hallmark of cancer, and the B-cell lymphoma-2 (Bcl-2) family of proteins is pivotal to mediating the intrinsic pathway of this process. Recent advances have yielded both pan-Bcl-2 small molecule inhibitors (SMIs) that inhibit both the Bcl-2 and the Mcl-1 arm of the Bcl-2 family anti-apoptotic proteins, as well as selective SMIs to differentially target the two arms. Of these SMIs, ABT-263 (navitoclax), AT-101 [(-)-gossypol], and obatoclax (GX15-070) are currently in clinical trials for multiple cancers. While pan-Bcl-2 inhibitors such as AT-101 and obatoclax can be more toxic for inhibiting all members of the anti-apoptotic Bcl-2 family of proteins, resistance can quickly develop for ABT-263, a selective Bcl-2 inhibitor. In this article, we discuss the current status of Bcl-2 family SMIs in preclinical and clinical development. As Mcl-1 upregulation is a major mechanism of ABT-263 resistance, Mcl-1-specific inhibitors are expected to be efficacious both in combination/sequential treatments and as a single agent against cancers resistant to ABT-263.
Keywords: :
Introduction
Apoptosis is an orchestrated cellular process important for maintaining the homeostasis between cell proliferation and cell death, and is also pivotal for the removal of diseased, damaged, and inflamed cells.Citation1 Apoptosis can be induced in two distinct pathways, both of which lead to the activation of effector caspases. The extrinsic pathway of apoptosis is initiated at the plasma membrane upon ligation of death receptors, leading to the subsequent activation of effector caspases. These caspases then execute the apoptotic pathway to trigger DNA fragmentation and membrane blebbing, two hallmarks of apoptosis.Citation2 The intrinsic pathway is initiated in response to intracellular stress, eventually leading to mitochondrial outer membrane permeabilization (MOMP), releasing factors including cytochrome c and Smac to activate downstream caspases.Citation3
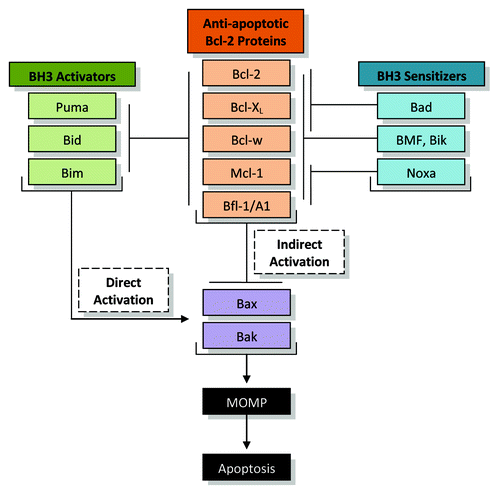
The B-cell lymphoma-2 (Bcl-2) family of proteins is central to the intrinsic pathway of apoptosis. The Bcl-2 proteins share one of four Bcl-2 homology (BH) domains, BH1–4, and of those, the BH3 domain is critical for mediating interactions among the family members.Citation4,Citation5 The Bcl-2 family of proteins can be grouped as either pro-apoptotic or anti-apoptotic. The pro-apoptotic group can be further divided as either multi-BH-domain proteins, including Bax and Bak, or as BH3-only proteins, such as Bid, Bad, Bim, Puma, and Noxa. The anti-apoptotic group of Bcl-2 proteins consists of Bcl-2, Bcl-XL, Bcl-w, Mcl-1, and Bfl-1/A1.Citation6 Two models account for how the various proteins interact to induce apoptosis (). In the indirect activation model, the anti-apoptotic Bcl-2 proteins physically interact and sequester Bak or Bax. Upon apoptotic stimuli, BH3-only proteins release Bak or Bax by binding and neutralizing the anti-apoptotic Bcl-2 proteins. In the direct activation model, anti-apoptotic Bcl-2 proteins function by sequestering particular BH3-only proteins called BH3 activators, including Bim, Bid, and Puma. Upon apoptotic stimuli, BH3 sensitizers such as Noxa or Bad liberate the activators, allowing them to activate Bak and Bax.Citation4,Citation7 The two models converge when activated Bak and Bax oligomerize, leading to MOMP and subsequent apoptosis. Therefore, the carefully regulated balance between pro-apoptotic and anti-apoptotic members of the Bcl-2 family determine the survival of the cell. It has repeatedly been shown that the overexpression or loss of pro- or anti-apoptotic members of the Bcl-2 proteins render cells correspondingly susceptible or resistant to apoptosis.Citation3,Citation8-Citation11 Therefore, deregulated apoptosis can be detrimental to the cell, tissue, or organ.
Figure 1. Regulation of apoptosis by the Bcl-2 family. The anti-apoptotic Bcl-2 protein family members include Bcl-2, Bcl-XL, Bcl-w, Mcl-1, and Bfl-1/A1. In the indirect activation model, the anti-apoptotic proteins sequester Bax and Bak through physical interactions. The upregulation of BH3-only proteins will disrupt this interaction, releasing Bax and Bak to oligomerize, allowing mitochondrial outer membrane permeabilization (MOMP), the release of key factors such as cytochrome c, and the induction of apoptosis. In the direct activation model, anti-apoptotic Bcl-2 protein members sequester BH3 activators including Puma, Bid, and Bim. BH3 sensitizers are able to disrupt this interaction, and the released activators directly activate Bax and Bak to allow MOMP and apoptosis. It is most likely that these two models happen in tandem.
Deregulated apoptosis is a hallmark of cancer.Citation12 The anti-apoptotic group of Bcl-2 family proteins is frequently found to be overexpressed in numerous cancers, causing both evasion of apoptosis and resistance to treatment.Citation3,Citation13-Citation18 Furthermore, since most anti-cancer drugs induce apoptosis through the intrinsic pathway, targeting the Bcl-2 family of proteins with small molecule inhibitors (SMIs) is especially attractive clinically either in single agent therapy or combination treatment.Citation2 While numerous compounds with anti-cancer activity have been shown to antagonize Bcl-2 family of proteins, such as epigallocatenin-3-gallate (EGCG) and chelerythrine, SMIs selective for the anti-apoptotic Bcl-2 proteins have only recently been identified.Citation19-Citation24 The first Bcl-2 selective SMI, HA14–1, was identified through a virtual screen in 2000. Due to the recent advances in crystallizing Bcl-XL and its complex with the Bak BH3 domain peptide, a hydrophobic pocket that interacts with other BH3 domains was identified and taken advantage of to aid drug design.Citation25,Citation26 The Bcl-2 protein structure was then modeled and HA14–1 was discovered through a computer-aided screen.Citation27 Following this, the fluorescence polarization assay (FPA) was soon developed to allow experimental high-throughput screening for SMIs of anti-apoptotic family proteins. This assay uses fluorescein-conjugated BH3 peptides and recombinant anti-apoptotic Bcl-2 proteins.Citation28 Using this method, several pan-Bcl-2 inhibitors with moderate affinity were discovered, including BH3–1, gossypol, and obatoclax.Citation23,Citation29,Citation30 Recently, structure-based design has also emerged to synthesize numerous high-affinity Bcl-2 SMIs. Using the NMR-guided fragment-based technology, low-affinity molecules, which bind to distinct sites on the hydrophobic groove of the Bcl-XL protein, were linked together to generate the high-affinity antagonist ABT-737.Citation22 Further rational design approaches have been successful at generating ABT-737 based high-affinity Bcl-2 SMIs, including compounds B-11 and compound 21.Citation31,Citation32 Moreover, the rational design of terphenyl-based Bak-BH3 α-helical proteomimetics resulted in the discovery of BH3-M6, a pan-Bcl-2 inhibitor that can inhibit both Bcl-XL and Mcl-1 to induce caspase dependent apoptosis.Citation33 Other developmental efforts have also been fruitful, resulting in the discovery of the selective Mcl-1 antagonist maritoclax, and the high-affinity pan-Bcl-2 inhibitor sabutoclax.Citation24,Citation34 Currently, three Bcl-2 inhibitors are undergoing clinical trials: ABT-737’s orally bioavailable analog ABT-263 (navitoclax), AT-101 [(-)-gossypol], and obatoclax (GX15–070).
Selectivity of Bcl-2 Inhibitors
Numerous compounds have been specifically developed or identified as Bcl-2 inhibitors. These compounds include ABT-737 and ABT-263, obatoclax, gossypol derivatives such as apogossypol, apogossypolone, and sabutoclax, other rational design-based compounds such as B-12 and BH3-M6, and the recently identified maritoclax.Citation22-Citation24,Citation31,Citation34-Citation37 However, the selectivity and affinities of these compounds for the anti-apoptotic Bcl-2 family members differ greatly among the different compounds as well as between different assays (). The difficulty in obtaining precise values for the affinity of Bcl-2 SMIs can be attributed to the affinity differences among the peptides used for the FPA. For example, ABT-737’s apparent affinity was 4-fold lower for Bcl-2, while 50-fold higher for Bcl-w, when using a BID-peptide compared with using a BIM-peptide.Citation21,Citation38 Therefore interpretations of affinity data for Bcl-2 SMIs should be taken with a grain of salt.
Figure 2. The selectivity of Bcl-2 SMIs. For each drug, green indicates relatively high affinity for the protein, orange indicates relatively low affinity for the protein, and red indicates no affinity for the protein was detected. Black indicates a lack of published data. The colors assigned were based on the drug’s affinities relative to the affinity of ABT-737 against Bcl-2, as well as on the relative affinities between the drug’s targets.
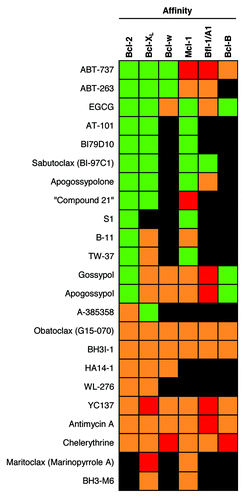
The selectivity of the Bcl-2 SMIs for the anti-apoptotic members differ as well. While agents such as obatoclax and AT-101 are classified as pan-Bcl-2 inhibitors due to its moderate affinity for all anti-apoptotic members, ABT-737 and its analog ABT-263 are selective for Bcl-2, Bcl-XL, and Bcl-w as they are modeled after the BH3-only protein Bad.Citation22 It is also the most potent of the inhibitors so far possessing affinities in the sub-nanomolar range. Other selective inhibitors have also been recently developed. A-385358 was rationally designed to be almost 100-fold more selective for Bcl-XL over Bcl-2, and is the most selective inhibitor for Bcl-XL to date. A-385358 was able to more potently kill Bcl-XL-dependent compared with Bcl-2-dependent lymphoma cells.Citation39
Maritoclax, also known as marinopyrrole A, was recently discovered as a Mcl-1-specific inhibitor through the screening of small compound libraries. Maritoclax is a natural product from a marine-derived species of StreptomycesCitation40 which binds to Mcl-1 with comparable affinity as obatoclax; however, unlike obatoclax, maritoclax does not bind to Bcl-XL. Maritoclax induces Mcl-1 proteasomal degradation and shows similar or greater in vitro efficacy toward Mcl-1 dependent cells as obatoclax. However, in cells dependent on Bcl-2 or Bcl-XL for survival, maritoclax demonstrates only marginal activity. Importantly, unlike obatoclax, which induces cell death indiscriminately in healthy peripheral blood mononuclear cells (PBMCs) and large granular lymphocyte leukemia (LGLL), maritoclax is significantly less effective against healthy PBMCs compared with LGLL cells. This suggests that maritoclax may be safer for treatment compared with pan-Bcl-2 inhibitors.Citation24 Interestingly, maritoclax has been shown to bind actin in addition to Mcl-1.Citation41 However, maritoclax induces cell death selectively in Mcl-1 dependent cells in a Bax/Bak dependent manner, indicating that maritoclax acts through the intrinsic pathway of apoptosis. Moreover, there is a positive correlation between the sensitivity to maritoclax and Mcl-1 expression in primary LGLL cells.Citation24 Lastly, actin is known to bind to and sequester the pro-apoptotic BH3-only protein Bmf, which is released to sensitize the cell to apoptosis upon actin depolymerization.Citation42,Citation43 Since Bmf interacts with not only Mcl-1 but also Bcl-2 and Bcl-XL, liberation of Bmf from actin should induce cell death in both Mcl-1 and Bcl-2/Bcl-XL dependent cells.Citation42 However, given that maritoclax only kills Mcl-1 dependent cells, it is unlikely that maritoclax induces apoptosis in a mechanism mediated by actin.
Since it is difficult for SMIs with micromolar affinities to reach physiologically relevant concentrations in vivo, such compounds are often deemed to be useful only as experimental tools. However, some compounds with moderate affinity, such as obatoclax and AT-101, have already demonstrated efficacy against cancer in clinical trials.Citation44,Citation45 Therefore, it is possible that these agents may act as non-selective Bcl-2 inhibitors, killing cancer cells through multiple pathways. In an important study by Vogler et al., it was shown that between obatoclax, gossypol, and ABT-737, only ABT-737 was unable to kill Bax/Bak double knockout cells.Citation46 Since Bax/Bak oligomerization is necessary for Bcl-2 mediated apoptosis, the ability of obatoclax and gossypol to induce cell death in these cells signifies its actions on other cellular pathways. It was similarly shown that obatoclax does not induce cytochrome c release from isolated mitochondria at physiologically relevant levels.Citation47 Given the inhibitory effect of Bcl-2 on Beclin-1, the mammalian homolog of the autophagy related gene 6 (Atg6), Bcl-2 SMIs often induce autophagy by disrupting Bcl-2 and Beclin-1 interactions.Citation48-Citation52 However, numerous reports have demonstrated Beclin-1 independent induction of autophagy by gossypol and obatoclax, possibly causing autophagic cell death independent of Bcl-2 interactions.Citation50,Citation53 Other reports have also shown obatoclax and gossypol to possess activities unrelated to Bcl-2 which may contribute to cell killing, such as reactive oxygen species generation or anion transport.Citation54-Citation57 Therefore, ABT-263 is possibly the only compound in clinical trials to selectively induce Bcl-2 dependent cell death.
Clinical Relevance of Bcl-2 Inhibitors
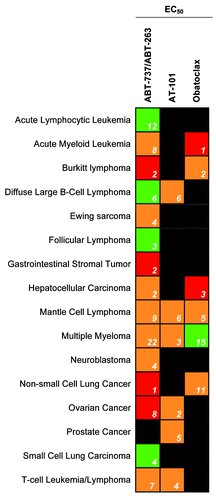
All three Bcl-2 SMIs in clinical trials, ABT-263, AT-101, and obatoclax, demonstrate remarkable preclinical efficacy in a variety of cell systems (). Notably, these Bcl-2 SMIs kill chemoresistant cell lines as efficaciously as susceptible cell lines, demonstrating a distinct mechanism of action and clinical promise.Citation58-Citation63
Figure 3. The potency of Bcl-2 SMIs currently in clinical trials against various cancer cells in culture. Green indicates high potency for the cancer type with an average EC50 value below 1 µM, orange indicates a moderate potency with an average EC50 value between 1 and 10 µM, and red indicates a low potency with an average EC50 value above 10 µM. Black indicates that no published EC50 exists for that drug and cancer type. The white numbers inside the box signify the number of experiments examined to obtain the average.
Bcl-2 inhibitors also synergize with a large number of other cancer treatments. All three Bcl-2 SMIs currently in clinical trials have shown to potentiate and synergize with chemotherapy and radiation in vitro.Citation22,Citation64-Citation72 Interestingly, mitogen-activated protein kinase (MAPK) pathway inhibitors, such as the epidermal growth factor receptor (EGFR) inhibitor gefitinib and erlotinib or the MEK inhibitor UO126 synergize with Bcl-2 SMIs.Citation73-Citation76 While the MAPK pathway is frequently upregulated in cancers and is therapeutic target by itself, synergy between Bcl-2 inhibitors and MAPK pathway inhibitors may actually be due to crosstalk between the two pathways. It has been shown that EGFR activation leads to Stat3 activation, which in turn increases the expression of Bcl-2 and Bcl-XL.Citation77,Citation78 Therefore, the inhibition of the MAPK pathway reduces the expression of Bcl-2 and Bcl-XL, while Bcl-2 inhibitors physically antagonize their anti-apoptotic actions to create a synergistic effect. Recently, ABT-737 has also been shown to possess immunosuppressive properties, possessing activity on lymphocytes and mast cells.Citation79-Citation82
Due to the selectivity of ABT-737 on specific Bcl-2 family protein members, resistance can develop rapidly. As ABT-737 disrupts Bcl-2/Bim interactions more readily compared with Bcl-XL/Bim, Bcl-XL overexpressing cancer cells can be resistant to ABT-737 treatment.Citation83,Citation84 The primary pathway for ABT-737 resistance appears to be Mcl-1 upregulation, as it is not targeted by the compound. It was repeatedly observed in a large number of cancer cell lines that Mcl-1 expression levels inversely correlate with ABT-737 susceptibility.Citation38,Citation59,Citation65,Citation85-Citation90 Human B-cell lymphoma cell lines derived from long-term exposure to ABT-737 were resistant to its treatment through a significant increase in Mcl-1 transcription and protein levels. The use of flavopiridol, a cyclin-dependent kinase 9 (CDK9) inhibitor shown to downregulate Mcl-1, was able to restore ABT-737 sensitivity.Citation91 Furthermore, melanoma, which is typically resistant to ABT-737 treatment, can be rendered susceptible to the compound through genetic or non-selective pharmacologic inhibition of Mcl-1 in multiple reports.Citation92-Citation95 Obatoclax has demonstrated significant antagonistic activity against Mcl-1, and has been used experimentally to demonstrate Mcl-1 mediated ABT-737 resistance as well.Citation96-Citation98 As the induction of the MAPK pathway also upregulates Mcl-1, the synergy between ABT-737 and MAPK pathway inhibitors can be due to its downregulation of Mcl-1.Citation99 Following the development of the first selective Mcl-1 inhibitor, maritoclax was immediately shown to be efficacious as a single agent in ABT-737 resistant, Mcl-1 dependent cell lines, and was strongly synergistic with ABT-737.Citation24 Overexpression of Noxa in multiple cancer systems have shown to restore drug susceptibility that is similar to Mcl-1 downregulation, mechanistically demonstrating that sequestration of pro-apoptotic Bcl-2 proteins by Mcl-1 can mediate drug resistance.Citation94,Citation100-Citation102 Recently it was also found that Mcl-1 can also be phosphorylated in response to ABT-737 to increase its interaction with Bim to prevent apoptosis.Citation101
The in vivo activities of these Bcl-2 SMIs were similarly promising. ABT-737 was shown to be extremely efficacious as a single agent in SCLC mouse xenograft models, as the compound was able to induce complete regression of tumors and prevent its regrowth for at least 2 months after the cessation of therapy.Citation22 ABT-737 by itself has also been shown to be effective in xenograft models for glioblastoma, multiple myeloma, acute myeloid leukemia, chronic myeloid leukemia, acute lymphoblastic leukemia, among others.Citation38,Citation65,Citation103-Citation105 Furthermore, the pan-Bcl-2 inhibitor AT-101 was shown to be efficacious in prostate cancer xenografts in different cell line models, resulting in tumor growth reductions.Citation106,Citation107 In another study, however, AT-101 was ineffective in a similar prostate cancer xenograft model, but was active in potentiating the effects of docetaxel.Citation108 Obatoclax has also been shown to potentiate other cancer treatments in xenograft models.Citation109,Citation110
All Bcl-2 SMIs in clinical trials have published Phase II results. ABT-263 (navitoclax), which is being developed by Abbott Laboratories and Genentech, was shown to be the most efficacious against chronic lymphocytic leukemia (CLL) in Phase I trials, with all 7 patients in one trial and 9 out of 21 patients with relapsed or refractory disease experiencing a partial response with median progression-free survival of more than a year.Citation111,Citation112 It was observed in these patients that Mcl-1 expression inversely correlated with response to drug, matching previous preclinical observations.Citation112 The recently published Phase II clinical trial data for ABT-263 indicate that it lacks efficacy in patients with relapsed small cell lung carcinoma (SCLC), as only 1 out of 39 patients experienced a partial response, and 36 patients withdrew due to disease progression or adverse events.Citation113 Currently multiple clinical trials are still also underway to assess the efficacy of ABT-263 in combination treatments.Citation114
Since Bcl-XL is important for platelet survival, thrombocytopenia was observed across all clinical trials for ABT-263.Citation115,Citation116 However, the platelet decreases were all observed to be transient and reversible upon treatment termination, and has not been associated with clinically significant bleeding.Citation111-Citation113,Citation117 Other hematological toxicities including neutropenia have also been observed, and could present significant problems when treating patients with hematological malignancies with an already compromised bone marrow reserve.Citation112
AT-101 is in the pipeline for Ascenta Therapeutics and has only demonstrated moderate efficacy in one Phase I/II clinical trial against castration-resistant prostate cancer. In this trial, two of 24 patients experienced a partial decrease in prostate-specific antigen release, while no objective partial responses were observed. However, 4 individuals unexpectedly developed small intestinal obstructions possibly related to the drug, causing discontinuation of treatment.Citation44 In a following larger clinical trial in combination with docetaxel and prednisone, however, efficacy was not met against castration-resistant prostate cancer.Citation118 AT-101 did not manage to meet efficacy endpoints both as a single agent against nor in combination with topotecan or docetaxel/prednisone against SCLC.Citation119-Citation121 Gastrointestinal toxicities were observed across all clinical trials with single-agent trials, but were not increased in combination treatments.
Obatoclax is developed by Gemin X Pharmaceuticals. As it is not orally bioavailable, treatment must be administered through infusion. Unlike ABT-263, obatoclax demonstrated moderate to no efficacy against hematological malignancies in Phase I trials. However, in these clinical trials where obatoclax was either infused for one hour or three hours, significant neural adverse events were observed, including somnolence, euphoria, and ataxia. It was observed that a three-hour infusion was necessary to reduce the occurrence of toxic events.Citation45,Citation122-Citation124 In a phase I trial, obatoclax demonstrated significant efficacy against SCLC as a single agent, where 2 of 8 patients with SCLC had partial response and 3 experienced stable disease.Citation125 Moreover, another Phase I clinical trial in combination with carboplatin and etoposide against extensive-stage SCLC was conducted. Obatoclax demonstrated efficacy in combination treatments to improve outcomes.Citation126 However, obatoclax in combination with topotecan against relapsed SCLC did not meet efficacy endpoints.Citation127 Another Phase II trial also indicates obatoclax to be ineffective toward myelofibrosis, despite the disease’s dependence on the Bcl-2 family anti-apoptotic proteins.Citation128
Future Perspectives
As clinical trial data for Bcl-2 SMIs continue to be published, we will likely gain more insight on the efficacy of these inhibitors against cancer. So far, clinical trials show promise for ABT-263 against CLL, and obatoclax in combination treatments against SCLC. However, numerous clinical trials have demonstrated a lack of efficacy where it was expected, such as AT-101 against prostate cancer. A higher affinity pan Bcl-2 inhibitor sabutoclax (BI-97C1) has recently been developed, and may yield clinical promise as it possesses nanomolar affinities for all of Mcl-1, Bcl-2, and Bcl-XL proteins, and induces cell death in a Bax/Bak-dependent manner. Moreover, it has demonstrated both in vitro and in vivo xenograft model efficacy against Bcl-2 dependent and Mcl-1 dependent prostate cancer cell lines.Citation34 Currently, research efforts have began focusing on different dosing regimens using Bcl-2 SMIs to improve efficacy.Citation129 Metronomic dosing, the uninterrupted use of a low dose of compound, have been suggested for Bcl-2 inhibitors including AT-101 and TW-37.Citation130,Citation131 It is hypothesized that the metronomic dosing of Bcl-2 inhibitors is able to more effectively induce growth arrest by targeting the tumor microenvironment. Sequential dosing of multiple therapies also shows preclinical promise. The sequential use of bortezomib and HA14–1 was more efficacious than both single agent and combination treatment regimens in in vitro models of multiple myeloma.Citation132 Sequential dosing may be particularly useful for ABT-263, as it cannot be administered for extended periods of time due to its severe toxicity on platelets. Moreover, both preclinical and clinical data have indicated the importance of Mcl-1 mediated ABT-263 resistance. As maritoclax is strongly synergistic with ABT-737, the sequential use of ABT-263 with Mcl-1 selective inhibitors may be particularly useful both to prevent the development of resistant cell populations and to allow the recovery of platelets in patients.Citation24
High-affinity pan Bcl-2 inhibitors could cause much toxicity in the patient, as numerous healthy cells depend on the maintenance of anti-apoptotic Bcl-2 family members for survival. Thus selective Bcl-2 SMIs demonstrate a distinct advantage in drug safety, although selectivity can also lead to resistance, as was seen in the case of ABT-263 and ABT-737. As simultaneous treatment of patients with multiple Bcl-2 inhibitors is imprudent due to toxicity reasons, it may be more sensible to use sequential treatments in order to minimize undesired side effects.
Maritoclax is expected to demonstrate single-agent efficacy in addition to combination therapies. It is known that the downregulation of Mcl-1 itself is efficacious against numerous cancer cells to mediate both cell killing and drug sensitivity.Citation110,Citation133-Citation136 Importantly, experiments show that multiple cancers, including multiple myeloma and acute myeloid leukemia, are highly dependent on Mcl-1 to sustain growth.Citation134,Citation137,Citation138 As ABT-737 does not target Mcl-1, these cancer cell lines are resistant to ABT-737 killing. For example, downregulation of Mcl-1 is able to completely kill acute myeloid leukemia cells in both cell culture and in vivo mouse models.Citation134 Following optimization, more Mcl-1 inhibitors with higher selectivity and affinity are likely to be generated for clinical use.
The Bcl-2 family proteins play a significant role in maintaining survival for a large number of cancers. SMIs for the anti-apoptotic proteins have already demonstrated that cancer cells can be killed through this distinct mechanism of action despite their resistance status to previous therapies. These inhibitors have demonstrated efficacy both as single agents and in combination therapy to improve patient outcome. However, selectivity, toxicity, resistance, and bioavailability remain a challenge for many of these compounds. Further efforts should focus on reducing undesired toxicities from these drugs through dosing or combination therapies, as well as chemical advances in synthesizing more selective Bcl-2 inhibitors.
Acknowledgments
We thank Yoshinori Takahashi and Megan Young for critical reading of the manuscript. This work is supported by a NIH/NCI grant (CA82197).
References
- Wong RS. Apoptosis in cancer: from pathogenesis to treatment. Journal of experimental & clinical cancer research. CR (East Lansing, Mich) 2011; 30:87
- Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 2006; 25:4798 - 811; http://dx.doi.org/10.1038/sj.onc.1209608; PMID: 16892092
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 2008; 9:47 - 59; http://dx.doi.org/10.1038/nrm2308; PMID: 18097445
- Giam M, Huang DC, Bouillet P. BH3-only proteins and their roles in programmed cell death. Oncogene 2008; 27:Suppl 1 S128 - 36; http://dx.doi.org/10.1038/onc.2009.50; PMID: 19641498
- Ni Chonghaile T, Letai A. Mimicking the BH3 domain to kill cancer cells. Oncogene 2008; 27:Suppl 1 S149 - 57; http://dx.doi.org/10.1038/onc.2009.52; PMID: 19641500
- Kelly PN, Strasser A. The role of Bcl-2 and its pro-survival relatives in tumourigenesis and cancer therapy. Cell Death Differ 2011; 18:1414 - 24; http://dx.doi.org/10.1038/cdd.2011.17; PMID: 21415859
- Quinn BA, Dash R, Azab B, Sarkar S, Das SK, Kumar S, et al. Targeting Mcl-1 for the therapy of cancer. Expert Opin Investig Drugs 2011; 20:1397 - 411; http://dx.doi.org/10.1517/13543784.2011.609167; PMID: 21851287
- Bae IH, Park MJ, Yoon SH, Kang SW, Lee SS, Choi KM, et al. Bcl-w promotes gastric cancer cell invasion by inducing matrix metalloproteinase-2 expression via phosphoinositide 3-kinase, Akt, and Sp1. Cancer Res 2006; 66:4991 - 5; http://dx.doi.org/10.1158/0008-5472.CAN-05-4254; PMID: 16707418
- Campbell KJ, Bath ML, Turner ML, Vandenberg CJ, Bouillet P, Metcalf D, et al. Elevated Mcl-1 perturbs lymphopoiesis, promotes transformation of hematopoietic stem/progenitor cells, and enhances drug resistance. Blood 2010; 116:3197 - 207; http://dx.doi.org/10.1182/blood-2010-04-281071; PMID: 20631380
- Villunger A, Michalak EM, Coultas L, Müllauer F, Böck G, Ausserlechner MJ, et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science 2003; 302:1036 - 8; http://dx.doi.org/10.1126/science.1090072; PMID: 14500851
- Grillot DA, Merino R, Pena JC, Fanslow WC, Finkelman FD, Thompson CB, et al. bcl-x exhibits regulated expression during B cell development and activation and modulates lymphocyte survival in transgenic mice. J Exp Med 1996; 183:381 - 91; http://dx.doi.org/10.1084/jem.183.2.381; PMID: 8627151
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100:57 - 70; http://dx.doi.org/10.1016/S0092-8674(00)81683-9; PMID: 10647931
- Buggins AG, Pepper CJ. The role of Bcl-2 family proteins in chronic lymphocytic leukaemia. Leuk Res 2010; 34:837 - 42; http://dx.doi.org/10.1016/j.leukres.2010.03.011; PMID: 20359747
- Yang E, Korsmeyer SJ. Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood 1996; 88:386 - 401; PMID: 8695785
- Yunis JJ, Frizzera G, Oken MM, McKenna J, Theologides A, Arnesen M. Multiple recurrent genomic defects in follicular lymphoma. A possible model for cancer. N Engl J Med 1987; 316:79 - 84; http://dx.doi.org/10.1056/NEJM198701083160204; PMID: 3537802
- Reed JC. Bcl-2 family proteins: strategies for overcoming chemoresistance in cancer. Adv Pharmacol 1997; 41:501 - 32; http://dx.doi.org/10.1016/S1054-3589(08)61070-4; PMID: 9204157
- Reed JC, Miyashita T, Takayama S, Wang HG, Sato T, Krajewski S, et al. BCL-2 family proteins: regulators of cell death involved in the pathogenesis of cancer and resistance to therapy. J Cell Biochem 1996; 60:23 - 32; http://dx.doi.org/10.1002/(SICI)1097-4644(19960101)60:1<23::AID-JCB5>3.0.CO;2-5; PMID: 8825412
- Coultas L, Strasser A. The role of the Bcl-2 protein family in cancer. Semin Cancer Biol 2003; 13:115 - 23; http://dx.doi.org/10.1016/S1044-579X(02)00129-3; PMID: 12654255
- Chung LY, Cheung TC, Kong SK, Fung KP, Choy YM, Chan ZY, et al. Induction of apoptosis by green tea catechins in human prostate cancer DU145 cells. Life Sci 2001; 68:1207 - 14; http://dx.doi.org/10.1016/S0024-3205(00)01020-1; PMID: 11228105
- Chan SL, Lee MC, Tan KO, Yang LK, Lee AS, Flotow H, et al. Identification of chelerythrine as an inhibitor of BclXL function. J Biol Chem 2003; 278:20453 - 6; http://dx.doi.org/10.1074/jbc.C300138200; PMID: 12702731
- Zhai D, Jin C, Satterthwait AC, Reed JC. Comparison of chemical inhibitors of antiapoptotic Bcl-2-family proteins. Cell Death Differ 2006; 13:1419 - 21; http://dx.doi.org/10.1038/sj.cdd.4401937; PMID: 16645636
- Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 2005; 435:677 - 81; http://dx.doi.org/10.1038/nature03579; PMID: 15902208
- Shore GC, Viallet J. Modulating the bcl-2 family of apoptosis suppressors for potential therapeutic benefit in cancer. Hematology Am Soc Hematol Educ Program 2005; •••:226 - 30; http://dx.doi.org/10.1182/asheducation-2005.1.226; PMID: 16304385
- Doi K, Li R, Sung SS, Wu H, Liu Y, Manieri W, et al. Discovery of marinopyrrole A (maritoclax) as a selective Mcl-1 antagonist that overcomes ABT-737 resistance by binding to and targeting Mcl-1 for proteasomal degradation. J Biol Chem 2012; 287:10224 - 35; http://dx.doi.org/10.1074/jbc.M111.334532; PMID: 22311987
- Muchmore SW, Sattler M, Liang H, Meadows RP, Harlan JE, Yoon HS, et al. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature 1996; 381:335 - 41; http://dx.doi.org/10.1038/381335a0; PMID: 8692274
- Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, et al. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science 1997; 275:983 - 6; http://dx.doi.org/10.1126/science.275.5302.983; PMID: 9020082
- Wang JL, Liu D, Zhang ZJ, Shan S, Han X, Srinivasula SM, et al. Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc Natl Acad Sci U S A 2000; 97:7124 - 9; http://dx.doi.org/10.1073/pnas.97.13.7124; PMID: 10860979
- Zhang H, Nimmer P, Rosenberg SH, Ng SC, Joseph M. Development of a high-throughput fluorescence polarization assay for Bcl-x(L). Anal Biochem 2002; 307:70 - 5; http://dx.doi.org/10.1016/S0003-2697(02)00028-3; PMID: 12137781
- Kitada S, Leone M, Sareth S, Zhai D, Reed JC, Pellecchia M. Discovery, characterization, and structure-activity relationships studies of proapoptotic polyphenols targeting B-cell lymphocyte/leukemia-2 proteins. J Med Chem 2003; 46:4259 - 64; http://dx.doi.org/10.1021/jm030190z; PMID: 13678404
- Degterev A, Lugovskoy A, Cardone M, Mulley B, Wagner G, Mitchison T, et al. Identification of small-molecule inhibitors of interaction between the BH3 domain and Bcl-xL. Nat Cell Biol 2001; 3:173 - 82; http://dx.doi.org/10.1038/35055085; PMID: 11175750
- Zheng CH, Yang H, Zhang M, Lu SH, Shi D, Wang J, et al. Design, synthesis, and activity evaluation of broad-spectrum small-molecule inhibitors of anti-apoptotic Bcl-2 family proteins: characteristics of broad-spectrum protein binding and its effects on anti-tumor activity. Bioorg Med Chem Lett 2012; 22:39 - 44; http://dx.doi.org/10.1016/j.bmcl.2011.11.101; PMID: 22172701
- Zhou H, Chen J, Meagher JL, Yang CY, Aguilar A, Liu L, et al. Design of Bcl-2 and Bcl-xL inhibitors with subnanomolar binding affinities based upon a new scaffold. J Med Chem 2012; 55:4664 - 82; http://dx.doi.org/10.1021/jm300178u; PMID: 22448988
- Kazi A, Sun J, Doi K, Sung SS, Takahashi Y, Yin H, et al. The BH3 α-helical mimic BH3-M6 disrupts Bcl-X(L), Bcl-2, and MCL-1 protein-protein interactions with Bax, Bak, Bad, or Bim and induces apoptosis in a Bax- and Bim-dependent manner. J Biol Chem 2011; 286:9382 - 92; http://dx.doi.org/10.1074/jbc.M110.203638; PMID: 21148306
- Wei J, Stebbins JL, Kitada S, Dash R, Placzek W, Rega MF, et al. BI-97C1, an optically pure Apogossypol derivative as pan-active inhibitor of antiapoptotic B-cell lymphoma/leukemia-2 (Bcl-2) family proteins. J Med Chem 2010; 53:4166 - 76; http://dx.doi.org/10.1021/jm1001265; PMID: 20443627
- Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res 2008; 68:3421 - 8; http://dx.doi.org/10.1158/0008-5472.CAN-07-5836; PMID: 18451170
- Becattini B, Kitada S, Leone M, Monosov E, Chandler S, Zhai D, et al. Rational design and real time, in-cell detection of the proapoptotic activity of a novel compound targeting Bcl-X(L). Chem Biol 2004; 11:389 - 95; http://dx.doi.org/10.1016/j.chembiol.2004.02.020; PMID: 15123268
- Sun Y, Wu J, Aboukameel A, Banerjee S, Arnold AA, Chen J, et al. Apogossypolone, a nonpeptidic small molecule inhibitor targeting Bcl-2 family proteins, effectively inhibits growth of diffuse large cell lymphoma cells in vitro and in vivo. Cancer Biol Ther 2008; 7:1418 - 26; http://dx.doi.org/10.4161/cbt.7.9.6430; PMID: 18769131
- Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell 2006; 10:375 - 88; http://dx.doi.org/10.1016/j.ccr.2006.10.006; PMID: 17097560
- Shoemaker AR, Oleksijew A, Bauch J, Belli BA, Borre T, Bruncko M, et al. A small-molecule inhibitor of Bcl-XL potentiates the activity of cytotoxic drugs in vitro and in vivo. Cancer Res 2006; 66:8731 - 9; http://dx.doi.org/10.1158/0008-5472.CAN-06-0367; PMID: 16951189
- Hughes CC, Prieto-Davo A, Jensen PR, Fenical W. The marinopyrroles, antibiotics of an unprecedented structure class from a marine Streptomyces sp. Org Lett 2008; 10:629 - 31; http://dx.doi.org/10.1021/ol702952n; PMID: 18205372
- Hughes CC, Yang YL, Liu WT, Dorrestein PC, La Clair JJ, Fenical W. Marinopyrrole A target elucidation by acyl dye transfer. J Am Chem Soc 2009; 131:12094 - 6; http://dx.doi.org/10.1021/ja903149u; PMID: 19673475
- Puthalakath H, Villunger A, O’Reilly LA, Beaumont JG, Coultas L, Cheney RE, et al. Bmf: a proapoptotic BH3-only protein regulated by interaction with the myosin V actin motor complex, activated by anoikis. Science 2001; 293:1829 - 32; http://dx.doi.org/10.1126/science.1062257; PMID: 11546872
- Schmelzle T, Mailleux AA, Overholtzer M, Carroll JS, Solimini NL, Lightcap ES, et al. Functional role and oncogene-regulated expression of the BH3-only factor Bmf in mammary epithelial anoikis and morphogenesis. Proc Natl Acad Sci U S A 2007; 104:3787 - 92; http://dx.doi.org/10.1073/pnas.0700115104; PMID: 17360431
- Liu G, Kelly WK, Wilding G, Leopold L, Brill K, Somer B. An open-label, multicenter, phase I/II study of single-agent AT-101 in men with castrate-resistant prostate cancer. Clin Cancer Res 2009; 15:3172 - 6; http://dx.doi.org/10.1158/1078-0432.CCR-08-2985; PMID: 19366825
- Schimmer AD, O’Brien S, Kantarjian H, Brandwein J, Cheson BD, Minden MD, et al. A phase I study of the pan bcl-2 family inhibitor obatoclax mesylate in patients with advanced hematologic malignancies. Clin Cancer Res 2008; 14:8295 - 301; http://dx.doi.org/10.1158/1078-0432.CCR-08-0999; PMID: 19088047
- Vogler M, Weber K, Dinsdale D, Schmitz I, Schulze-Osthoff K, Dyer MJ, et al. Different forms of cell death induced by putative BCL2 inhibitors. Cell Death Differ 2009; 16:1030 - 9; http://dx.doi.org/10.1038/cdd.2009.48; PMID: 19390557
- Buron N, Porceddu M, Brabant M, Desgué D, Racoeur C, Lassalle M, et al. Use of human cancer cell lines mitochondria to explore the mechanisms of BH3 peptides and ABT-737-induced mitochondrial membrane permeabilization. PLoS One 2010; 5:e9924; http://dx.doi.org/10.1371/journal.pone.0009924; PMID: 20360986
- Cao Y, Klionsky DJ. Physiological functions of Atg6/Beclin 1: a unique autophagy-related protein. Cell Res 2007; 17:839 - 49; http://dx.doi.org/10.1038/cr.2007.78; PMID: 17893711
- Malik SA, Shen S, Mariño G, BenYounès A, Maiuri MC, Kroemer G. BH3 mimetics reveal the network properties of autophagy-regulatory signaling cascades. Autophagy 2011; 7:914 - 6; http://dx.doi.org/10.4161/auto.7.8.15785; PMID: 21508685
- Gao P, Bauvy C, Souquère S, Tonelli G, Liu L, Zhu Y, et al. The Bcl-2 homology domain 3 mimetic gossypol induces both Beclin 1-dependent and Beclin 1-independent cytoprotective autophagy in cancer cells. J Biol Chem 2010; 285:25570 - 81; http://dx.doi.org/10.1074/jbc.M110.118125; PMID: 20529838
- Lian J, Karnak D, Xu L. The Bcl-2-Beclin 1 interaction in (-)-gossypol-induced autophagy versus apoptosis in prostate cancer cells. Autophagy 2010; 6:1201 - 3; http://dx.doi.org/10.4161/auto.6.8.13549; PMID: 20930561
- Lian J, Wu X, He F, Karnak D, Tang W, Meng Y, et al. A natural BH3 mimetic induces autophagy in apoptosis-resistant prostate cancer via modulating Bcl-2-Beclin1 interaction at endoplasmic reticulum. Cell Death Differ 2011; 18:60 - 71; http://dx.doi.org/10.1038/cdd.2010.74; PMID: 20577262
- McCoy F, Hurwitz J, McTavish N, Paul I, Barnes C, O’Hagan B, et al. Obatoclax induces Atg7-dependent autophagy independent of beclin-1 and BAX/BAK. Cell Death Dis 2010; 1:e108; http://dx.doi.org/10.1038/cddis.2010.86; PMID: 21368880
- Ko CH, Shen SC, Yang LY, Lin CW, Chen YC. Gossypol reduction of tumor growth through ROS-dependent mitochondria pathway in human colorectal carcinoma cells. Int J Cancer 2007; 121:1670 - 9; http://dx.doi.org/10.1002/ijc.22910; PMID: 17597109
- Díaz de Greñu B, Iglesias Hernández P, Espona M, Quiñonero D, Light ME, Torroba T, et al. Synthetic prodiginine obatoclax (GX15-070) and related analogues: anion binding, transmembrane transport, and cytotoxicity properties. Chemistry 2011; 17:14074 - 83; http://dx.doi.org/10.1002/chem.201101547; PMID: 22069220
- Zhang M, Liu H, Tian Z, Griffith BN, Ji M, Li QQ. Gossypol induces apoptosis in human PC-3 prostate cancer cells by modulating caspase-dependent and caspase-independent cell death pathways. Life Sci 2007; 80:767 - 74; http://dx.doi.org/10.1016/j.lfs.2006.11.004; PMID: 17156797
- Hu ZY, Wang J, Cheng G, Zhu XF, Huang P, Yang D, et al. Apogossypolone targets mitochondria and light enhances its anticancer activity by stimulating generation of singlet oxygen and reactive oxygen species. Chin J Cancer 2011; 30:41 - 53; http://dx.doi.org/10.5732/cjc.010.10295; PMID: 21192843
- Klymenko T, Brandenburg M, Morrow C, Dive C, Makin G. The novel Bcl-2 inhibitor ABT-737 is more effective in hypoxia and is able to reverse hypoxia-induced drug resistance in neuroblastoma cells. Mol Cancer Ther 2011; 10:2373 - 83; http://dx.doi.org/10.1158/1535-7163.MCT-11-0326; PMID: 22006676
- Chauhan D, Velankar M, Brahmandam M, Hideshima T, Podar K, Richardson P, et al. A novel Bcl-2/Bcl-X(L)/Bcl-w inhibitor ABT-737 as therapy in multiple myeloma. Oncogene 2007; 26:2374 - 80; http://dx.doi.org/10.1038/sj.onc.1210028; PMID: 17016430
- Martin AP, Park MA, Mitchell C, Walker T, Rahmani M, Thorburn A, et al. BCL-2 family inhibitors enhance histone deacetylase inhibitor and sorafenib lethality via autophagy and overcome blockade of the extrinsic pathway to facilitate killing. Mol Pharmacol 2009; 76:327 - 41; http://dx.doi.org/10.1124/mol.109.056309; PMID: 19483105
- Crawford N, Chacko AD, Savage KI, McCoy F, Redmond K, Longley DB, Fennell DA. Platinum resistant cancer cells conserve sensitivity to BH3 domains and obatoclax induced mitochondrial apoptosis. Apoptosis: an international journal on programmed cell death 2011; 16:311-20.
- Hu W, Wang F, Tang J, Liu X, Yuan Z, Nie C, et al. Proapoptotic protein Smac mediates apoptosis in cisplatin-resistant ovarian cancer cells when treated with the anti-tumor agent AT101. J Biol Chem 2012; 287:68 - 80; http://dx.doi.org/10.1074/jbc.M111.271205; PMID: 22052903
- Balakrishnan K, Burger JA, Wierda WG, Gandhi V. AT-101 induces apoptosis in CLL B cells and overcomes stromal cell-mediated Mcl-1 induction and drug resistance. Blood 2009; 113:149 - 53; http://dx.doi.org/10.1182/blood-2008-02-138560; PMID: 18836097
- Lieber J, Ellerkamp V, Wenz J, Kirchner B, Warmann SW, Fuchs J, et al. Apoptosis sensitizers enhance cytotoxicity in hepatoblastoma cells. Pediatr Surg Int 2012; 28:149 - 59; http://dx.doi.org/10.1007/s00383-011-2988-z; PMID: 21971946
- Tagscherer KE, Fassl A, Campos B, Farhadi M, Kraemer A, Böck BC, et al. Apoptosis-based treatment of glioblastomas with ABT-737, a novel small molecule inhibitor of Bcl-2 family proteins. Oncogene 2008; 27:6646 - 56; http://dx.doi.org/10.1038/onc.2008.259; PMID: 18663354
- Macoska JA, Adsule S, Tantivejkul K, Wang S, Pienta KJ, Lee CT. -(-)Gossypol promotes the apoptosis of bladder cancer cells in vitro. Pharmacological research: the official journal of the Italian Pharmacological Society 2008; 58:323-31.
- Paoluzzi L, Gonen M, Gardner JR, Mastrella J, Yang D, Holmlund J, et al. Targeting Bcl-2 family members with the BH3 mimetic AT-101 markedly enhances the therapeutic effects of chemotherapeutic agents in in vitro and in vivo models of B-cell lymphoma. Blood 2008; 111:5350 - 8; http://dx.doi.org/10.1182/blood-2007-12-129833; PMID: 18292288
- Zerp SF, Stoter R, Kuipers G, Yang D, Lippman ME, van Blitterswijk WJ, et al. AT-101, a small molecule inhibitor of anti-apoptotic Bcl-2 family members, activates the SAPK/JNK pathway and enhances radiation-induced apoptosis. Radiat Oncol 2009; 4:47; http://dx.doi.org/10.1186/1748-717X-4-47; PMID: 19852810
- Moretti L, Li B, Kim KW, Chen H, Lu B. AT-101, a pan-Bcl-2 inhibitor, leads to radiosensitization of non-small cell lung cancer. J Thorac Oncol 2010; 5:680 - 7; PMID: 20354451
- Pan J, Cheng C, Verstovsek S, Chen Q, Jin Y, Cao Q. The BH3-mimetic GX15-070 induces autophagy, potentiates the cytotoxicity of carboplatin and 5-fluorouracil in esophageal carcinoma cells. Cancer Lett 2010; 293:167 - 74; http://dx.doi.org/10.1016/j.canlet.2010.01.006; PMID: 20153924
- Brem EA, Thudium K, Khubchandani S, Tsai PC, Olejniczak SH, Bhat S, et al. Distinct cellular and therapeutic effects of obatoclax in rituximab-sensitive and -resistant lymphomas. Br J Haematol 2011; 153:599 - 611; http://dx.doi.org/10.1111/j.1365-2141.2011.08669.x; PMID: 21492126
- Zhang C, Cai TY, Zhu H, Yang LQ, Jiang H, Dong XW, et al. Synergistic antitumor activity of gemcitabine and ABT-737 in vitro and in vivo through disrupting the interaction of USP9X and Mcl-1. Mol Cancer Ther 2011; 10:1264 - 75; http://dx.doi.org/10.1158/1535-7163.MCT-10-1091; PMID: 21566062
- Cragg MS, Jansen ES, Cook M, Harris C, Strasser A, Scott CL. Treatment of B-RAF mutant human tumor cells with a MEK inhibitor requires Bim and is enhanced by a BH3 mimetic. J Clin Invest 2008; 118:3651 - 9; http://dx.doi.org/10.1172/JCI35437; PMID: 18949058
- Cragg MS, Kuroda J, Puthalakath H, Huang DC, Strasser A. Gefitinib-induced killing of NSCLC cell lines expressing mutant EGFR requires BIM and can be enhanced by BH3 mimetics. PLoS medicine 2007; 4:1681-89; discussion 90.
- Boehm AL, Sen M, Seethala R, Gooding WE, Freilino M, Wong SM, et al. Combined targeting of epidermal growth factor receptor, signal transducer and activator of transcription-3, and Bcl-X(L) enhances antitumor effects in squamous cell carcinoma of the head and neck. Mol Pharmacol 2008; 73:1632 - 42; http://dx.doi.org/10.1124/mol.107.044636; PMID: 18326051
- Witters LM, Witkoski A, Planas-Silva MD, Berger M, Viallet J, Lipton A. Synergistic inhibition of breast cancer cell lines with a dual inhibitor of EGFR-HER-2/neu and a Bcl-2 inhibitor. Oncol Rep 2007; 17:465 - 9; PMID: 17203189
- Grandis JR, Drenning SD, Zeng Q, Watkins SC, Melhem MF, Endo S, et al. Constitutive activation of Stat3 signaling abrogates apoptosis in squamous cell carcinogenesis in vivo. Proc Natl Acad Sci U S A 2000; 97:4227 - 32; http://dx.doi.org/10.1073/pnas.97.8.4227; PMID: 10760290
- Masuda M, Suzui M, Weinstein IB. Effects of epigallocatechin-3-gallate on growth, epidermal growth factor receptor signaling pathways, gene expression, and chemosensitivity in human head and neck squamous cell carcinoma cell lines. Clin Cancer Res 2001; 7:4220 - 9; PMID: 11751523
- Bardwell PD, Gu J, McCarthy D, Wallace C, Bryant S, Goess C, et al. The Bcl-2 family antagonist ABT-737 significantly inhibits multiple animal models of autoimmunity. J Immunol 2009; 182:7482 - 9; http://dx.doi.org/10.4049/jimmunol.0802813; PMID: 19494271
- Carrington EM, Vikstrom IB, Light A, Sutherland RM, Londrigan SL, Mason KD, et al. BH3 mimetics antagonizing restricted prosurvival Bcl-2 proteins represent another class of selective immune modulatory drugs. Proc Natl Acad Sci U S A 2010; 107:10967 - 71; http://dx.doi.org/10.1073/pnas.1005256107; PMID: 20534453
- Cippà PE, Kraus AK, Lindenmeyer MT, Chen J, Guimezanes A, Bardwell PD, et al. Resistance to ABT-737 in activated T lymphocytes: molecular mechanisms and reversibility by inhibition of the calcineurin-NFAT pathway. Cell Death Dis 2012; 3:e299; http://dx.doi.org/10.1038/cddis.2012.38; PMID: 22513873
- Karlberg M, Ekoff M, Huang DC, Mustonen P, Harvima IT, Nilsson G. The BH3-mimetic ABT-737 induces mast cell apoptosis in vitro and in vivo: potential for therapeutics. J Immunol 2010; 185:2555 - 62; http://dx.doi.org/10.4049/jimmunol.0903656; PMID: 20639495
- Goldsmith KC, Gross M, Peirce S, Luyindula D, Liu X, Vu A, et al. Mitochondrial Bcl-2 family dynamics define therapy response and resistance in neuroblastoma. Cancer Res 2012; 72:2565 - 77; http://dx.doi.org/10.1158/0008-5472.CAN-11-3603; PMID: 22589275
- Mérino D, Khaw SL, Glaser SP, Anderson DJ, Belmont LD, Wong C, et al. Bcl-2, Bcl-x(L), and Bcl-w are not equivalent targets of ABT-737 and navitoclax (ABT-263) in lymphoid and leukemic cells. Blood 2012; 119:5807 - 16; http://dx.doi.org/10.1182/blood-2011-12-400929; PMID: 22538851
- Lam LT, Lu X, Zhang H, Lesniewski R, Rosenberg S, Semizarov D. A microRNA screen to identify modulators of sensitivity to BCL2 inhibitor ABT-263 (navitoclax). Mol Cancer Ther 2010; 9:2943 - 50; http://dx.doi.org/10.1158/1535-7163.MCT-10-0427; PMID: 20829195
- Tahir SK, Wass J, Joseph MK, Devanarayan V, Hessler P, Zhang H, et al. Identification of expression signatures predictive of sensitivity to the Bcl-2 family member inhibitor ABT-263 in small cell lung carcinoma and leukemia/lymphoma cell lines. Mol Cancer Ther 2010; 9:545 - 57; http://dx.doi.org/10.1158/1535-7163.MCT-09-0651; PMID: 20179162
- Wesarg E, Hoffarth S, Wiewrodt R, Kröll M, Biesterfeld S, Huber C, et al. Targeting BCL-2 family proteins to overcome drug resistance in non-small cell lung cancer. Int J Cancer 2007; 121:2387 - 94; http://dx.doi.org/10.1002/ijc.22977; PMID: 17688235
- Al-Harbi S, Hill BT, Mazumder S, Singh K, Devecchio J, Choudhary G, et al. An antiapoptotic BCL-2 family expression index predicts the response of chronic lymphocytic leukemia to ABT-737. Blood 2011; 118:3579 - 90; http://dx.doi.org/10.1182/blood-2011-03-340364; PMID: 21772052
- Bodet L, Gomez-Bougie P, Touzeau C, Dousset C, Descamps G, Maïga S, et al. ABT-737 is highly effective against molecular subgroups of multiple myeloma. Blood 2011; 118:3901 - 10; http://dx.doi.org/10.1182/blood-2010-11-317438; PMID: 21835956
- Touzeau C, Dousset C, Bodet L, Gomez-Bougie P, Bonnaud S, Moreau A, et al. ABT-737 induces apoptosis in mantle cell lymphoma cells with a Bcl-2high/Mcl-1low profile and synergizes with other antineoplastic agents. Clin Cancer Res 2011; 17:5973 - 81; http://dx.doi.org/10.1158/1078-0432.CCR-11-0955; PMID: 21821698
- Yecies D, Carlson NE, Deng J, Letai A. Acquired resistance to ABT-737 in lymphoma cells that up-regulate MCL-1 and BFL-1. Blood 2010; 115:3304 - 13; http://dx.doi.org/10.1182/blood-2009-07-233304; PMID: 20197552
- Keuling AM, Felton KE, Parker AA, Akbari M, Andrew SE, Tron VA. RNA silencing of Mcl-1 enhances ABT-737-mediated apoptosis in melanoma: role for a caspase-8-dependent pathway. PLoS One 2009; 4:e6651; http://dx.doi.org/10.1371/journal.pone.0006651; PMID: 19684859
- Weber A, Kirejczyk Z, Potthoff S, Ploner C, Hacker G. Endogenous Noxa Determines the Strong Proapoptotic Synergism of the BH3-Mimetic ABT-737 with Chemotherapeutic Agents in Human Melanoma Cells. Translational oncology 2009; 2:73-83.
- Lucas KM, Mohana-Kumaran N, Lau D, Zhang XD, Hersey P, Huang DC, et al. Modulation of NOXA and MCL-1 as a strategy for sensitizing melanoma cells to the BH3-mimetic ABT-737. Clin Cancer Res 2012; 18:783 - 95; http://dx.doi.org/10.1158/1078-0432.CCR-11-1166; PMID: 22173547
- Chen J, Zhang X, Lentz C, Abi-Daoud M, Paré GC, Yang X, et al. miR-193b Regulates Mcl-1 in Melanoma. Am J Pathol 2011; 179:2162 - 8; http://dx.doi.org/10.1016/j.ajpath.2011.07.010; PMID: 21893020
- Mott JL, Bronk SF, Mesa RA, Kaufmann SH, Gores GJ. BH3-only protein mimetic obatoclax sensitizes cholangiocarcinoma cells to Apo2L/TRAIL-induced apoptosis. Mol Cancer Ther 2008; 7:2339 - 47; http://dx.doi.org/10.1158/1535-7163.MCT-08-0285; PMID: 18723481
- Jiang CC, Wroblewski D, Yang F, Hersey P, Zhang XD. Human melanoma cells under endoplasmic reticulum stress are more susceptible to apoptosis induced by the BH3 mimetic obatoclax. Neoplasia 2009; 11:945 - 55; PMID: 19724688
- Nguyen M, Marcellus RC, Roulston A, Watson M, Serfass L, Murthy Madiraju SR, et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci U S A 2007; 104:19512 - 7; http://dx.doi.org/10.1073/pnas.0709443104; PMID: 18040043
- Fassl A, Tagscherer KE, Richter J, Berriel Diaz M, Alcantara Llaguno SR, Campos B, et al. Notch1 signaling promotes survival of glioblastoma cells via EGFR-mediated induction of anti-apoptotic Mcl-1. Oncogene 2012; http://dx.doi.org/10.1038/onc.2011.615; PMID: 22249262
- Yamaguchi R, Perkins G. Mcl-1 levels need not be lowered for cells to be sensitized for ABT-263/737-induced apoptosis. Cell Death Dis 2011; 2:e227; http://dx.doi.org/10.1038/cddis.2011.109; PMID: 22071632
- Mazumder S, Choudhary GS, Al-Harbi S, Almasan A. Mcl-1 Phosphorylation defines ABT-737 resistance that can be overcome by increased NOXA expression in leukemic B cells. Cancer Res 2012; 72:3069 - 79; http://dx.doi.org/10.1158/0008-5472.CAN-11-4106; PMID: 22525702
- Zall H, Weber A, Besch R, Zantl N, Häcker G. Chemotherapeutic drugs sensitize human renal cell carcinoma cells to ABT-737 by a mechanism involving the Noxa-dependent inactivation of Mcl-1 or A1. Mol Cancer 2010; 9:164; http://dx.doi.org/10.1186/1476-4598-9-164; PMID: 20576107
- Trudel S, Stewart AK, Li Z, Shu Y, Liang SB, Trieu Y, et al. The Bcl-2 family protein inhibitor, ABT-737, has substantial antimyeloma activity and shows synergistic effect with dexamethasone and melphalan. Clin Cancer Res 2007; 13:621 - 9; http://dx.doi.org/10.1158/1078-0432.CCR-06-1526; PMID: 17255285
- Kuroda J, Kimura S, Andreeff M, Ashihara E, Kamitsuji Y, Yokota A, et al. ABT-737 is a useful component of combinatory chemotherapies for chronic myeloid leukaemias with diverse drug-resistance mechanisms. Br J Haematol 2008; 140:181 - 90; PMID: 18028486
- Lock R, Carol H, Houghton PJ, Morton CL, Kolb EA, Gorlick R, et al. Initial testing (stage 1) of the BH3 mimetic ABT-263 by the pediatric preclinical testing program. Pediatr Blood Cancer 2008; 50:1181 - 9; http://dx.doi.org/10.1002/pbc.21433; PMID: 18085673
- Loberg RD, McGregor N, Ying C, Sargent E, Pienta KJ. In vivo evaluation of AT-101 (R-(-)-gossypol acetic acid) in androgen-independent growth of VCaP prostate cancer cells in combination with surgical castration. Neoplasia 2007; 9:1030 - 7; http://dx.doi.org/10.1593/neo.07778; PMID: 18084610
- Zhang XQ, Huang XF, Mu SJ, An QX, Xia AJ, Chen R, et al. Inhibition of proliferation of prostate cancer cell line, PC-3, in vitro and in vivo using (-)-gossypol. Asian J Androl 2010; 12:390 - 9; http://dx.doi.org/10.1038/aja.2009.87; PMID: 20081872
- Meng Y, Tang W, Dai Y, Wu X, Liu M, Ji Q, et al. Natural BH3 mimetic (-)-gossypol chemosensitizes human prostate cancer via Bcl-xL inhibition accompanied by increase of Puma and Noxa. Mol Cancer Ther 2008; 7:2192 - 202; http://dx.doi.org/10.1158/1535-7163.MCT-08-0333; PMID: 18645028
- Dasmahapatra G, Lembersky D, Son MP, Patel H, Peterson D, Attkisson E, et al. Obatoclax interacts synergistically with the irreversible proteasome inhibitor carfilzomib in GC- and ABC-DLBCL cells in vitro and in vivo. Mol Cancer Ther 2012; 11:1122 - 32; http://dx.doi.org/10.1158/1535-7163.MCT-12-0021; PMID: 22411899
- Mitchell C, Yacoub A, Hossein H, Martin AP, Bareford MD, Eulitt P, et al. Inhibition of MCL-1 in breast cancer cells promotes cell death in vitro and in vivo. Cancer Biol Ther 2010; 10:903 - 17; http://dx.doi.org/10.4161/cbt.10.9.13273; PMID: 20855960
- Wilson WH, O’Connor OA, Czuczman MS, LaCasce AS, Gerecitano JF, Leonard JP, et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol 2010; 11:1149 - 59; http://dx.doi.org/10.1016/S1470-2045(10)70261-8; PMID: 21094089
- Roberts AW, Seymour JF, Brown JR, Wierda WG, Kipps TJ, Khaw SL, Carney DA, He SZ, Huang DC, Xiong H, Cui Y, Busman TA, McKeegan EM, Krivoshik AP, Enschede SH, Humerickhouse R. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 2012; 30:488-96.
- Rudin CM, Hann CL, Garon EB, Ribeiro de Oliveira M, Bonomi PD, Camidge DR, et al. Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin Cancer Res 2012; 18:3163 - 9; http://dx.doi.org/10.1158/1078-0432.CCR-11-3090; PMID: 22496272
- ClinicalTrials.gov. U.S. National Institute of Health.
- Zhang H, Nimmer PM, Tahir SK, Chen J, Fryer RM, Hahn KR, et al. Bcl-2 family proteins are essential for platelet survival. Cell Death Differ 2007; 14:943 - 51; PMID: 17205078
- Schoenwaelder SM, Jarman KE, Gardiner EE, Hua M, Qiao J, White MJ, et al. Bcl-xL-inhibitory BH3 mimetics can induce a transient thrombocytopathy that undermines the hemostatic function of platelets. Blood 2011; 118:1663 - 74; http://dx.doi.org/10.1182/blood-2011-04-347849; PMID: 21673344
- Gandhi L, Camidge DR, Ribeiro de Oliveira M, Bonomi P, Gandara D, Khaira D, Hann CL, McKeegan EM, Litvinovich E, Hemken PM, Dive C, Enschede SH, Nolan C, Chiu YL, Busman T, Xiong H, Krivoshik AP, Humerickhouse R, Shapiro GI, Rudin CM. Phase I study of Navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 2011; 29:909-16.
- Sonpavde G, Matveev V, Burke JM, Caton JR, Fleming MT, Hutson TE, Galsky MD, Berry WR, Karlov P, Holmlund JT, Wood BA, Brookes M, Leopold L. Randomized phase II trial of docetaxel plus prednisone in combination with placebo or AT-101, an oral small molecule Bcl-2 family antagonist, as first-line therapy for metastatic castration-resistant prostate cancer. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO 2012.
- Heist RS, Fain J, Chinnasami B, Khan W, Molina JR, Sequist LV, et al. Phase I/II study of AT-101 with topotecan in relapsed and refractory small cell lung cancer. J Thorac Oncol 2010; 5:1637 - 43; http://dx.doi.org/10.1097/JTO.0b013e3181e8f4dc; PMID: 20808253
- Ready N, Karaseva NA, Orlov SV, Luft AV, Popovych O, Holmlund JT, et al. Double-blind, placebo-controlled, randomized phase 2 study of the proapoptotic agent AT-101 plus docetaxel, in second-line non-small cell lung cancer. J Thorac Oncol 2011; 6:781 - 5; http://dx.doi.org/10.1097/JTO.0b013e31820a0ea6; PMID: 21289522
- Baggstrom MQ, Qi Y, Koczywas M, Argiris A, Johnson EA, Millward MJ, et al, Mayo Phase 2 Consortium, California Consortium. A phase II study of AT-101 (Gossypol) in chemotherapy-sensitive recurrent extensive-stage small cell lung cancer. J Thorac Oncol 2011; 6:1757 - 60; http://dx.doi.org/10.1097/JTO.0b013e31822e2941; PMID: 21918390
- O’Brien SM, Claxton DF, Crump M, Faderl S, Kipps T, Keating MJ, et al. Phase I study of obatoclax mesylate (GX15-070), a small molecule pan-Bcl-2 family antagonist, in patients with advanced chronic lymphocytic leukemia. Blood 2009; 113:299 - 305; http://dx.doi.org/10.1182/blood-2008-02-137943; PMID: 18931344
- Hwang JJ, Kuruvilla J, Mendelson D, Pishvaian MJ, Deeken JF, Siu LL, et al. Phase I dose finding studies of obatoclax (GX15-070), a small molecule pan-BCL-2 family antagonist, in patients with advanced solid tumors or lymphoma. Clin Cancer Res 2010; 16:4038 - 45; http://dx.doi.org/10.1158/1078-0432.CCR-10-0822; PMID: 20538761
- Oki Y, Copeland A, Hagemeister F, Fayad LE, Fanale M, Romaguera J, et al. Experience with obatoclax mesylate (GX15-070), a small molecule pan-Bcl-2 family antagonist in patients with relapsed or refractory classical Hodgkin lymphoma. Blood 2012; 119:2171 - 2; http://dx.doi.org/10.1182/blood-2011-11-391037; PMID: 22383790
- Paik PK, Rudin CM, Brown A, Rizvi NA, Takebe N, Travis W, et al. A phase I study of obatoclax mesylate, a Bcl-2 antagonist, plus topotecan in solid tumor malignancies. Cancer Chemother Pharmacol 2010; 66:1079 - 85; http://dx.doi.org/10.1007/s00280-010-1265-5; PMID: 20165849
- Chiappori AA, Schreeder MT, Moezi MM, Stephenson JJ, Blakely J, Salgia R, et al. A phase I trial of pan-Bcl-2 antagonist obatoclax administered as a 3-h or a 24-h infusion in combination with carboplatin and etoposide in patients with extensive-stage small cell lung cancer. Br J Cancer 2012; 106:839 - 45; http://dx.doi.org/10.1038/bjc.2012.21; PMID: 22333598
- Paik PK, Rudin CM, Pietanza MC, Brown A, Rizvi NA, Takebe N, et al. A phase II study of obatoclax mesylate, a Bcl-2 antagonist, plus topotecan in relapsed small cell lung cancer. Lung Cancer 2011; 74:481 - 5; http://dx.doi.org/10.1016/j.lungcan.2011.05.005; PMID: 21620511
- Parikh SA, Kantarjian H, Schimmer A, Walsh W, Asatiani E, El-Shami K, et al. Phase II study of obatoclax mesylate (GX15-070), a small-molecule BCL-2 family antagonist, for patients with myelofibrosis. Clin Lymphoma Myeloma Leuk 2010; 10:285 - 9; http://dx.doi.org/10.3816/CLML.2010.n.059; PMID: 20709666
- Zhang Z, Wu G, Gao J, Song T. Inclusion complex of a Bcl-2 inhibitor with cyclodextrin: characterization, cellular accumulation, and in vivo antitumor activity. Mol Pharm 2010; 7:1348 - 54; http://dx.doi.org/10.1021/mp100081x; PMID: 20550194
- Imai A, Zeitlin BD, Visioli F, Dong Z, Zhang Z, Krishnamurthy S, et al. Metronomic dosing of BH3 mimetic small molecule yields robust antiangiogenic and antitumor effects. Cancer Res 2012; 72:716 - 25; http://dx.doi.org/10.1158/0008-5472.CAN-10-2873; PMID: 22158856
- Zeitlin BD, Spalding AC, Campos MS, Ashimori N, Dong Z, Wang S, et al. Metronomic small molecule inhibitor of Bcl-2 (TW-37) is antiangiogenic and potentiates the antitumor effect of ionizing radiation. Int J Radiat Oncol Biol Phys 2010; 78:879 - 87; http://dx.doi.org/10.1016/j.ijrobp.2010.04.024; PMID: 20675079
- Pei XY, Dai Y, Grant S. The proteasome inhibitor bortezomib promotes mitochondrial injury and apoptosis induced by the small molecule Bcl-2 inhibitor HA14-1 in multiple myeloma cells. Leukemia: official journal of the Leukemia Society of America. Leukemia Research Fund, UK 2003; 17:2036 - 45; http://dx.doi.org/10.1038/sj.leu.2403109
- Dash R, Azab B, Quinn BA, Shen X, Wang XY, Das SK, et al. Apogossypol derivative BI-97C1 (Sabutoclax) targeting Mcl-1 sensitizes prostate cancer cells to mda-7/IL-24-mediated toxicity. Proc Natl Acad Sci U S A 2011; 108:8785 - 90; http://dx.doi.org/10.1073/pnas.1100769108; PMID: 21555592
- Glaser SP, Lee EF, Trounson E, Bouillet P, Wei A, Fairlie WD, et al. Anti-apoptotic Mcl-1 is essential for the development and sustained growth of acute myeloid leukemia. Genes Dev 2012; 26:120 - 5; http://dx.doi.org/10.1101/gad.182980.111; PMID: 22279045
- Martin AP, Mitchell C, Rahmani M, Nephew KP, Grant S, Dent P. Inhibition of MCL-1 enhances lapatinib toxicity and overcomes lapatinib resistance via BAK-dependent autophagy. Cancer Biol Ther 2009; 8:2084 - 96; http://dx.doi.org/10.4161/cbt.8.21.9895; PMID: 19823038
- Cruickshanks N, Hamed HA, Bareford MD, Poklepovic A, Fisher PB, Grant S, et al. Lapatinib and obatoclax kill tumor cells through blockade of ERBB1/3/4 and through inhibition of BCL-XL and MCL-1. Mol Pharmacol 2012; 81:748 - 58; http://dx.doi.org/10.1124/mol.112.077586; PMID: 22357666
- Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, et al. The landscape of somatic copy-number alteration across human cancers. Nature 2010; 463:899 - 905; http://dx.doi.org/10.1038/nature08822; PMID: 20164920
- Tiedemann RE, Zhu YX, Schmidt J, Shi CX, Sereduk C, Yin H, et al. Identification of molecular vulnerabilities in human multiple myeloma cells by RNA interference lethality screening of the druggable genome. Cancer Res 2012; 72:757 - 68; http://dx.doi.org/10.1158/0008-5472.CAN-11-2781; PMID: 22147262