Abstract
Filopodia are dynamic, actin-rich finger-like structures that protrude from the cell membrane and play important roles in cell migration and neurite initiation and outgrowth. The insulin receptor substrate protein of 53 kDa (IRSp53) and the mammalian Diaphanous members of the formin family of proteins (mDia) are two key players in the formation of filopodia and neurites. IRSp53 is an adaptor protein that acts at the membrane:actin interface, coupling membrane deformation with F-actin polymerization. mDia formin proteins, instead, can nucleate and polymerize linear actin filaments. Emerging genetic and biochemical evidence indicate that there are multiple and independent pathways leading to filopodium and neurite formation, but the precise molecular components of these pathways remain ill-defined. We recently identified the PDZ domain-containing protein LIN7 as a novel regulator of IRSp53. We further showed that the association between these two proteins is required to promote the formation of filopodia and neurites independently from mDia formin proteins, highlighting novel mechanisms of filopodia and neurite formation.
Keywords: :
Filopodia are dynamic actin-rich cell surface protrusions involved in cell migration, axon guidance, and wound healing.Citation1 Plasma membrane protrusion and actin dynamics are essential events for filopodium formation and, by coupling these two events, the insulin receptor substrate protein of 53 kDa (IRSp53) is a key player in this process.Citation2-Citation5 Its N-terminal inverse bin-amphiphysin-Rvs (I-BAR) domain binds to and deforms the plasma membrane, whereas its C-terminal Src homology 3 (SH3) domain interacts with various actin regulators, including the mammalian Diaphanous (mDia) isoforms mDia1 and mDia2.Citation6 In the inactive state, the SH3 domain of IRSp53 has been suggested to be locked by intramolecular interactions. Binding of the Rho GTPase Cdc42 to a partial Cdc42/Rac interactive binding motif (CRIB) located between the I-BAR and SH3 domains may activate IRSp53 by unmasking the SH3 domain.Citation7
The Diaphanous mDia1 and mDia2 members of the formin family of proteins are known for their ability to nucleate and polymerize linear actin filaments. Both of these formins have been linked to filopodium formation downstream of the Rho GTPase Rif.Citation8,Citation9 Silencing of mDia1 reduced the number of filopodia induced by IRSp53, while silencing of mDia2 did not affect IRSp53-induced filopodium formation.Citation9 These data suggest that mDia1 may induce filopodium protrusions either by binding IRSp53 or independently, whereas mDia2 always functions in pathways independent from IRSp53.
We have recently demonstrated that LIN7, a PDZ protein interactor of IRSp53, is a regulator of filopodia induced by IRSp53. We showed that the formation of filopodia containing actin filaments along their entire length depends on motifs contained in LIN7 and IRSp53 that mediate their association and filopodia tip localization. We further demonstrated that the coexpression of LIN7 with IRSp53 enhances the formation of filopodia in neuronal cells. In addition, downregulation of LIN7 inhibited differentiation of neuroblastoma N2A cells. A highly significant reduction of neurites was measured in LIN7-silenced cells, and neuritogenesis was rescued by RNAi-resistant full length LIN7 or a chimeric LIN7-IRSp53 fusion protein. In contrast, LIN7 mutants lacking domains for association with IRSp53 (PDZ domain) or with plasma membrane protein complexes (L27 domain) failed to rescue neuritogenesis. Taken together, our data indicate that stable filopodia and neurite outgrowth depends on the IRSp53 association with LIN7.Citation10
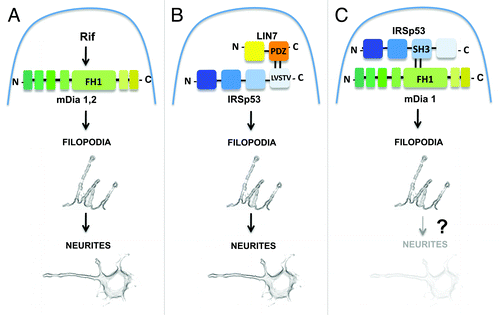
Evidence indicate that neurites form by the dilation of stable filopodia.Citation11 Our data showing that LIN7 acts in the stabilization of actin filaments along filopodia indicate the presence of a pathway dependent on LIN7 and IRSp53 for neuritogenesis. However, the removal of LIN7 had no effects on filopodia and neurite formation induced by either mDia2 or mDia1, since both formins, when individually overexpressed in LIN7-silenced cells, were sufficient to restore completely neuritogenesis.Citation10 Particularly surprising were the results obtained by the ectopic expression of mDia1 in LIN7-silenced neuronal cells, since the specific need of this formin in the formation of filopodia promoted by IRSp53 has recently been well documented.Citation12 However, it must be pointed out that more than one independent pathway for filopodium and therefore neurite formation exist.Citation1 Our data are consistent with this notion, indicating that a LIN7-IRSp53 pathway may act independently of mDia1 and mDia2. In neuronal N2A cells, the LIN7-IRSp53 pathway may co-exist with the Rif-mDia1/mDia2 pathway and with the one dependent on IRSp53 and mDia1 (see ). Notably, the latter pathway has been demonstrated in a neuroblastoma cell line strictly related to the N2A cell line, although no data are available concerning the possibility that the IRSp53-mDia1 pathway, together with filopodia formation, may also regulate neuritogenesis. Further experiments will be required to elucidate the nature of these pathways and to understand the LIN7 functions in filopodia and neurite formation.
Figure 1. A model describing parallel pathways for the formation of filopodia and neurites. (A) mDia1,2-dependent pathway.Citation9 Activation of mDia1 or mDia2-mediated by the Rho GTPase Rif induces filopodia formation and neuritogenesis; (B) LIN7-IRSp53-dependent pathway.Citation10 Binding between the class I PDZ domain of LIN7 and the PDZ target motif (LVSTV) of IRSp53 induces the formation of filopodia and neurites; (C) IRSp53-mDia1-dependent pathway.Citation12 Binding of mDia1 to the SH3 domain of IRSp53 induces the formation of filopodia. No data are available concerning a role of this pathway in neuritogenesis. Domains for protein-protein interaction are indicated.
References
- Mattila PK, Lappalainen P. Filopodia: molecular architecture and cellular functions. Nat Rev Mol Cell Biol 2008; 9:446 - 54; http://dx.doi.org/10.1038/nrm2406; PMID: 18464790
- Ahmed S, Goh WI, Bu W. I-BAR domains, IRSp53 and filopodium formation. Semin Cell Dev Biol 2010; 21:350 - 6; http://dx.doi.org/10.1016/j.semcdb.2009.11.008; PMID: 19913105
- Govind S, Kozma R, Monfries C, Lim L, Ahmed S. Cdc42Hs facilitates cytoskeletal reorganization and neurite outgrowth by localizing the 58-kD insulin receptor substrate to filamentous actin. J Cell Biol 2001; 152:579 - 94; http://dx.doi.org/10.1083/jcb.152.3.579; PMID: 11157984
- Disanza A, Mantoani S, Hertzog M, Gerboth S, Frittoli E, Steffen A, et al. Regulation of cell shape by Cdc42 is mediated by the synergic actin-bundling activity of the Eps8-IRSp53 complex. Nat Cell Biol 2006; 8:1337 - 47; http://dx.doi.org/10.1038/ncb1502; PMID: 17115031
- Yamagishi A, Masuda M, Ohki T, Onishi H, Mochizuki N. A novel actin bundling/filopodium-forming domain conserved in insulin receptor tyrosine kinase substrate p53 and missing in metastasis protein. J Biol Chem 2004; 279:14929 - 36; http://dx.doi.org/10.1074/jbc.M309408200; PMID: 14752106
- Faix J, Grosse R. Staying in shape with formins. Dev Cell 2006; 10:693 - 706; http://dx.doi.org/10.1016/j.devcel.2006.05.001; PMID: 16740473
- Lim KB, Bu W, Goh WI, Koh E, Ong SH, Pawson T, et al. The Cdc42 effector IRSp53 generates filopodia by coupling membrane protrusion with actin dynamics. J Biol Chem 2008; 283:20454 - 72; http://dx.doi.org/10.1074/jbc.M710185200; PMID: 18448434
- Pellegrin S, Mellor H. The Rho family GTPase Rif induces filopodia through mDia2. Curr Biol 2005; 15:129 - 33; http://dx.doi.org/10.1016/j.cub.2005.01.011; PMID: 15668168
- Goh WI, Sudhaharan T, Lim KB, Sem KP, Lau CL, Ahmed S. Rif-mDia1 interaction is involved in filopodium formation independent of Cdc42 and Rac effectors. J Biol Chem 2011; 286:13681 - 94; http://dx.doi.org/10.1074/jbc.M110.182683; PMID: 21339294
- Crespi A, Ferrari I, Lonati P, Disanza A, Fornasari D, Scita G, et al. LIN7 regulates the filopodium- and neurite-promoting activity of IRSp53. J Cell Sci 2012; http://dx.doi.org/10.1242/jcs.106484; PMID: 22767515
- Dent EW, Kwiatkowski AV, Mebane LM, Philippar U, Barzik M, Rubinson DA, et al. Filopodia are required for cortical neurite initiation. Nat Cell Biol 2007; 9:1347 - 59; http://dx.doi.org/10.1038/ncb1654; PMID: 18026093
- Goh WI, Lim KB, Sudhaharan T, Sem KP, Bu W, Chou AM, et al. mDia1 and WAVE2 proteins interact directly with IRSp53 in filopodia and are involved in filopodium formation. J Biol Chem 2012; 287:4702 - 14; http://dx.doi.org/10.1074/jbc.M111.305102; PMID: 22179776