Abstract
Intracellular Flightless I (Flii), a gelsolin family member, has been found to have roles modulating actin regulation, transcriptional regulation and inflammation. In vivo Flii can regulate wound healing responses. We have recently shown that a pool of Flii is secreted by fibroblasts and macrophages, cells typically found in wounds, and its secretion can be upregulated upon wounding. We show that secreted Flii can bind to the bacterial cell wall component lipopolysaccharide and has the potential to regulate inflammation. We now show that secreted Flii is present in both acute and chronic wound fluid.
Introduction
Flightless I (Flii) is a multifunctional protein of the gelsolin family of actin-remodelling proteins. There are eight members of this family in mouse and humans, each containing one or two gelsolin domains consisting of three repeated gelsolin motifs of between 125 and 150 amino acids in length.Citation1 Flii contains 6 repeat gelsolin motifs and an additional 11 Leucine Rich Repeat (LRR) domains not present in other family members.Citation2,Citation3 It has been found to play roles in many processes including actin regulation, transcription and inflammation. Flii is distributed across many cellular compartments, as would be expected for such a multifunctional protein, including the nucleus, cytosol and lysosomes.Citation3 In vivo, Flii negatively regulates excisional wound, blister wound and burn injury repair.Citation4-Citation6 Flii overexpressing mice (FliiTg/Tg) have impaired healing with larger, less contracted wounds, reduced cell proliferation and delayed epithelial migration. In contrast, mice with reduced levels of Flii (Flii+/−) have improved wound healing with increased epithelial migration and enhanced wound contraction.Citation5 Wounds in Flii overexpressing mice also show significantly elevated levels of collagen I and overexpression of collagen is a major contributing factor to hypertrophic, or excessive scar formation.Citation5 Given Flii’s known role in processes that are all involved in regulating tissue repair it is not surprising that it could alter the course of wound healing and scar formation.
Regulation of Actin and Transcription
Unlike many gelsolin family members, which enhance actin polymerisation and cap actin filaments, Flii binds actin filaments and actin monomers and inhibits actin polymerisation.Citation7,Citation8 Flii caps but does not sever actin filaments and thereby retards actin filament turnover.Citation7,Citation8 Flii associates with focal adhesions, which are specialized structures that link the actin cytoskeleton to the surface integrin receptors and anchor cells to the extracellular matrix, and can alter their formation.Citation7,Citation9 Cells with reduced levels of gelsolin migrate slower, while a reduction in Flii levels leads to an increase in migration.Citation5 Both fibroblasts and keratinocytes, cells typically found in wounds, that have less Flii migrate faster in vitro and in vivo and vice versa.Citation7,Citation10 The impaired wound healing seen in mice overexpressing Flii could in part be due to the ability of Flii to inhibit cell adhesion and migration. However, Flii has several other functions, which could also contribute to its negative role in tissue repair. Like gelsolin, Flii has the ability to regulate transcription.Citation11 Flii binds to hormone-activated nuclear receptors, including the estrogen and thyroid hormone receptor, as well as the coactivators GRIP1and CARM1.Citation12 Flii positively regulates hormone-stimulated gene expression by the estrogen receptor through its gelsolin region and is involved in the recruitment of the chromatin remodelling complex SW1/SNF to estrogen-responsive promoters.Citation12,Citation13 Flii also inhibits β-catenin and LEF1/TCF-mediated transcription.Citation14 Thus, Flii has the ability to alter transcription in part through its gelsolin domains. Our new data has shown that in fibroblasts Flii is found in the nucleus of some but not all cells (Fig. One and 2)Citation3 and it is probably this pool of Flii which may well be responsible for regulating transcription in these cells.Citation15 Our new data further suggests that Flii may have distinct roles in different cell types or under specific conditions. We show that unlike fibroblasts, macrophages have little to no Flii in the nucleus whether they were activated or not suggesting that its roles could differ depending on cell type or stimulus.Citation3
Flii’s Role in Regulating Inflammation
In recent years it has becoming increasingly obvious that Flii has an important role in dampening inflammation.Citation3,Citation16-Citation19 Unlike other gelsolin family members Flii has 11 LRR domains in the N-terminus.Citation3 These LRR domains share nearly 50% similarity to the LRR domains of the immune related receptor Toll-like Receptor (TLR) 4. The immune system detects infection or injury via the LRR domains of TLRs.Citation20 They can bind to Pathogen-Associated Molecular Pattern (PAMPs) molecules, such as the gram-negative bacteria cell wall component lipopolysaccharide or to Damage-Associated Molecular Pattern (DAMPs) molecules such as HMGB1 that are released from damaged and dying cells, as well as extracellular matrix cleavage products.Citation21 Their binding in turn activates intracellular TLR signaling pathways that ultimately lead to the secretion of cytokines. Typically both DAMPs and PAMPs are present in wounds. The importance of these domains in Flii was first hinted at by mouse knockout studies of Flii and other gelsolin family members.Citation15 Apart from Flii, mice lacking members of the gelsolin family are viable, but with actin defects.Citation15 In contrast, homozygous disruption of the Flii gene in mice leads to very early failure of embryonic development with impaired cellularization and gastrulation of the embryo indicating that Flii is essential developmental regulator and has additional important functions, which could be related to the role of the LRR domains.Citation22
We have recently shown a pool of Flii is located in the cytosol and this pool may in part be responsible for its role in dampening inflammation.Citation3,Citation16,Citation19 Although inflammation appears to be a necessary part of the normal adult wound healing process, excessive activation of TLR receptors and the subsequent increased or prolonged inflammatory response can induce considerable tissue damage which can lead to impaired healing. Flii is upregulated in mouse wounds peaking around day 7 when the inflammatory stage of tissue repair is being switched off.Citation5 Whether Flii is playing a role in dampening inflammation during tissue repair has yet to be tested in vivo. However in vitro Flii has been shown to dampen inflammation in multiple ways. In macrophages Flii is located in a complex with the TLR adaptor protein MyD88 through its interaction with nucleoredoxin.Citation17,Citation19 Binding of Flii to this complex inhibits MyD88 binding to TLR4, which results in the inhibition TLR signaling pathways and reduces cytokine secretion.Citation17,Citation19 We recently found a pool of Flii localized to late endosomes/lysosomes in fibroblasts and macrophages where it may also have a role in dampening inflammation.Citation3,Citation18 Flii binds to caspase-1 and in doing so inhibits the maturation of the cytokine pro-interleukin-1β to pro-interleukin-1β in macrophages, thus reducing its secretion.Citation18 Exactly where in the cell pro-interleukin-1β is cleaved to form the mature secreted form is controversial; however, there is data to suggest it may take place in the lysosome and that the mature form can be secreted from this compartment.Citation23
Flii is Secreted and the Secreted Form also has the Potential to Alter Inflammation
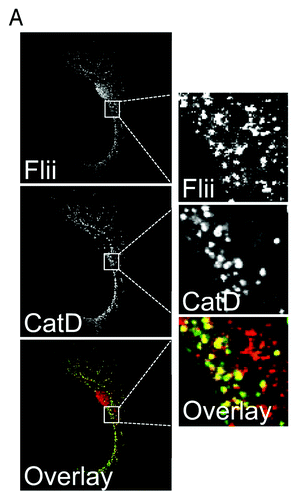
Flii was thought to be solely an intracellular protein, however, our recent study has shown that in vitro Flii is constitutively secreted by at least two cell types typically found in wounds; macrophages and fibroblasts.Citation3 This secretion from fibroblasts can be upregulated in response to wounding and by macrophages in response to LPS stimulation.Citation3 The upregulation in secretion after wounding suggest this pool of Flii may play a role during the repair process. In macrophages Flii localizes to late endosomes/lysosomes but not to compartments typically associated with the classical secretory pathway, for example the Golgi complex in macrophages.Citation3 Similar results are shown here for primary fibroblasts ( and ). Flii does not colocate with the trans-Golgi network and recycling endosome-associated SNARE protein Vti1b in primary fibroblasts () or with the recycling endosome associated SNARE protein VAMP3 (). However, a pool of Flii colocalizes with the lysosomal enzyme Cathepsin D in these cells (). Our recent data shows that Flii is secreted from macrophages via late endosomes/lysosomes in a manner regulated by Rab7 and Stx11.Citation3 This data suggests that Flii may therefore not only affect cellular activities via its intracellular/nuclear functions but it may also have important extracellular activities.
Figure 1. Flii is not found in the classical secretory pathway in fibroblasts. Primary fibroblasts were fixed with methanol, immunostained for Flii (mouse anti-Flii antibody) in combination with the recycling endosome SNARE protein VAMP3 (A) or the trans-Golgi complex and recycling endosome SNARE Vti1b (B). Flii is not located in compartments of the classical secretory pathway in fibroblasts.
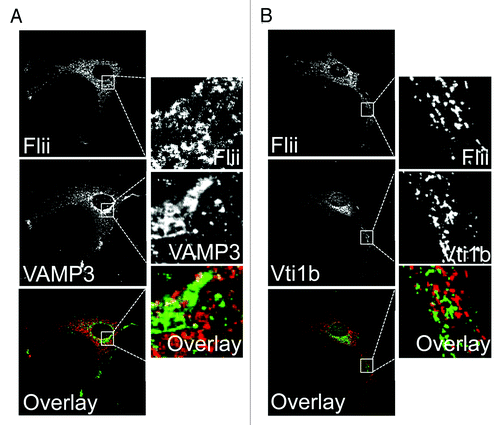
Figure 2. Flii is located in late endosomes/lysosomes in fibroblasts. (A) Primary fibroblasts were fixed with methanol, immunostained for Flii (mouse anti-Flii antibody) and the late endosomal/lysosomal enzyme cathepsin D (CatD). Flii co-localizes with cathepsin D in late endosomes/lysosomes in fibroblasts.
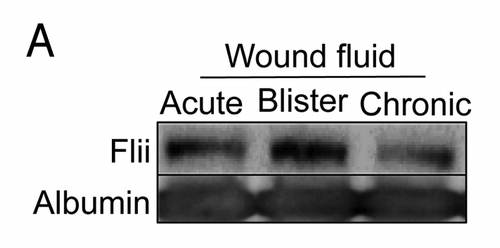
In vivo, we have shown that Flii is also present in human plasma samples.Citation3 Given Flii’s important role in wound healing we have now looked to see whether Flii is secreted into wound fluid from a number of sources. We find that secreted Flii is also present in acute wound fluid from patients undergoing abdominoplasty and in blister fluid as well as in chronic wound fluid taken from patients with venous leg ulcers (). Exactly what role Flii is playing in the wound fluid is currently unclear; however our results suggest that Flii can form a complex with the bacterial cell wall protein lipopolysaccharide (LPS).Citation3 An antibody to the first LLR domain is able to inhibit formation of this complex suggesting that the LRR region might be involved in this process.Citation3 Altering the level of secreted Flii in the media showed that Flii can negatively influence the LPS induced production and secretion of cytokine, such that cells stimulated with LPS in the presence of media with higher levels of Flii have reduced production and secretion of TNF.Citation3 Thus, it would appear that secreted Flii has the potential to dampen inflammation.
Figure 3. Flii is present in wound fluid from acute and chronic wounds. The clinical investigations were conducted under approval from the Women’s and Children’s Health Network Human Research Ethics Committee, Adelaide, Australia, in accordance to the Declaration of Helsinski principles and with written informed consent. (A) Wound fluid collected from patients undergoing abdominoplasty, from blister fluid and from patients with venous leg ulcers was immunoblotted for Flii and albumin. Flii is secreted into both acute and chronic wounds.
Prolonged or augmented inflammation can induce significant tissue damage unfavorable to the repair process thus the body has developed mechanisms that finely tune and regulate TLR activation. For example, surface TLR expression is downregulated after activation and at the same time soluble TLRs are secreted that compete with their membrane-associated forms for binding to ligands, thus limiting the stimulation of TLR signaling and inflammation. It is possible given the timing of Flii’s upregulation after injury that Flii could be potentially playing a role in limiting the inflammatory response both from within the cells and in the extracellular matrix. It will be interesting in the future to see whether this is the case.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by fellowships to R.Z.M. (#457247) and A.J.C. (#1002009) from the National Health and Medical Research Council of Australia and a University of Queensland International Postgraduate Research Scholarship to C.O.
References
- Kwiatkowski DJ, Janmey PA, Yin HL. Identification of critical functional and regulatory domains in gelsolin. J Cell Biol 1989; 108:1717 - 26; http://dx.doi.org/10.1083/jcb.108.5.1717; PMID: 2541138
- Kopecki Z, Cowin AJ. Flightless I: an actin-remodelling protein and an important negative regulator of wound repair. Int J Biochem Cell Biol 2008; 40:1415 - 9; http://dx.doi.org/10.1016/j.biocel.2007.04.011; PMID: 17526423
- Lei N, Franken L, Ruzehaji N, Offenhäuser C, Cowin AJ, Murray RZ. Flightless, secreted through a late endosome/lysosome pathway, binds LPS and dampens cytokine secretion. J Cell Sci 2012; In press http://dx.doi.org/10.1242/jcs.099507; PMID: 22718342
- Adams DH, Ruzehaji N, Strudwick XL, Greenwood JE, Campbell HD, Arkell R, et al. Attenuation of Flightless I, an actin-remodelling protein, improves burn injury repair via modulation of transforming growth factor (TGF)-beta1 and TGF-beta3. Br J Dermatol 2009; 161:326 - 36; http://dx.doi.org/10.1111/j.1365-2133.2009.09296.x; PMID: 19519830
- Cowin AJ, Adams DH, Strudwick XL, Chan H, Hooper JA, Sander GR, et al. Flightless I deficiency enhances wound repair by increasing cell migration and proliferation. J Pathol 2007; 211:572 - 81; http://dx.doi.org/10.1002/path.2143; PMID: 17326236
- Kopecki Z, Arkell RM, Strudwick XL, Hirose M, Ludwig RJ, Kern JS, et al. Overexpression of the Flii gene increases dermal-epidermal blistering in an autoimmune ColVII mouse model of epidermolysis bullosa acquisita. J Pathol 2011; 225:401 - 13; http://dx.doi.org/10.1002/path.2973; PMID: 21984127
- Mohammad I, Arora PD, Naghibzadeh Y, Wang Y, Li J, Mascarenhas W, et al. Flightless I is a focal adhesion-associated actin-capping protein that regulates cell migration. FASEB J 2012; 26:3260 - 72; http://dx.doi.org/10.1096/fj.11-202051; PMID: 22581781
- Silacci P, Mazzolai L, Gauci C, Stergiopulos N, Yin HL, Hayoz D. Gelsolin superfamily proteins: key regulators of cellular functions. Cell Mol Life Sci 2004; 61:2614 - 23; http://dx.doi.org/10.1007/s00018-004-4225-6; PMID: 15526166
- Kopecki Z, O’Neill GM, Arkell RM, Cowin AJ. Regulation of focal adhesions by flightless i involves inhibition of paxillin phosphorylation via a Rac1-dependent pathway. J Invest Dermatol 2011; 131:1450 - 9; http://dx.doi.org/10.1038/jid.2011.69; PMID: 21430700
- Kopecki Z, Arkell R, Powell BC, Cowin AJ. Flightless I regulates hemidesmosome formation and integrin-mediated cellular adhesion and migration during wound repair. J Invest Dermatol 2009; 129:2031 - 45; http://dx.doi.org/10.1038/jid.2008.461; PMID: 19212345
- Archer SK, Claudianos C, Campbell HD. Evolution of the gelsolin family of actin-binding proteins as novel transcriptional coactivators. Bioessays 2005; 27:388 - 96; http://dx.doi.org/10.1002/bies.20200; PMID: 15770676
- Lee YH, Campbell HD, Stallcup MR. Developmentally essential protein flightless I is a nuclear receptor coactivator with actin binding activity. Mol Cell Biol 2004; 24:2103 - 17; http://dx.doi.org/10.1128/MCB.24.5.2103-2117.2004; PMID: 14966289
- Jeong KW, Lee YH, Stallcup MR. Recruitment of the SWI/SNF chromatin remodeling complex to steroid hormone-regulated promoters by nuclear receptor coactivator flightless-I. J Biol Chem 2009; 284:29298 - 309; http://dx.doi.org/10.1074/jbc.M109.037010; PMID: 19720835
- Lee YH, Stallcup MR. Interplay of Fli-I and FLAP1 for regulation of beta-catenin dependent transcription. Nucleic Acids Res 2006; 34:5052 - 9; http://dx.doi.org/10.1093/nar/gkl652; PMID: 16990252
- Archer SK, Behm CA, Claudianos C, Campbell HD. The flightless I protein and the gelsolin family in nuclear hormone receptor-mediated signalling. Biochem Soc Trans 2004; 32:940 - 2; http://dx.doi.org/10.1042/BST0320940; PMID: 15506930
- Dai P, Jeong SY, Yu Y, Leng T, Wu W, Xie L, et al. Modulation of TLR signaling by multiple MyD88-interacting partners including leucine-rich repeat Fli-I-interacting proteins. J Immunol 2009; 182:3450 - 60; http://dx.doi.org/10.4049/jimmunol.0802260; PMID: 19265123
- Hayashi T, Funato Y, Terabayashi T, Morinaka A, Sakamoto R, Ichise H, et al. Nucleoredoxin negatively regulates Toll-like receptor 4 signaling via recruitment of flightless-I to myeloid differentiation primary response gene (88). J Biol Chem 2010; 285:18586 - 93; http://dx.doi.org/10.1074/jbc.M110.106468; PMID: 20400501
- Li J, Yin HL, Yuan J. Flightless-I regulates proinflammatory caspases by selectively modulating intracellular localization and caspase activity. J Cell Biol 2008; 181:321 - 33; http://dx.doi.org/10.1083/jcb.200711082; PMID: 18411310
- Wang T, Chuang TH, Ronni T, Gu S, Du YC, Cai H, et al. Flightless I homolog negatively modulates the TLR pathway. J Immunol 2006; 176:1355 - 62; PMID: 16424162
- Bell JK, Mullen GE, Leifer CA, Mazzoni A, Davies DR, Segal DM. Leucine-rich repeats and pathogen recognition in Toll-like receptors. Trends Immunol 2003; 24:528 - 33; http://dx.doi.org/10.1016/S1471-4906(03)00242-4; PMID: 14552836
- Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 2007; 81:1 - 5; http://dx.doi.org/10.1189/jlb.0306164; PMID: 17032697
- Campbell HD, Fountain S, McLennan IS, Berven LA, Crouch MF, Davy DA, et al. Fliih, a gelsolin-related cytoskeletal regulator essential for early mammalian embryonic development. Mol Cell Biol 2002; 22:3518 - 26; http://dx.doi.org/10.1128/MCB.22.10.3518-3526.2002; PMID: 11971982
- Eder C. Mechanisms of interleukin-1beta release. Immunobiology 2009; 214:543 - 53; http://dx.doi.org/10.1016/j.imbio.2008.11.007; PMID: 19250700