Abstract
The phenotype of smooth muscle cells is regulated by multiple environmental factors including mechanical forces. Mechanical stretch of mouse portal veins ex vivo has been shown to promote contractile differentiation by activation of the Rho-pathway, an effect that is dependent on the influx of calcium via L-type calcium channels. MicroRNAs have recently been demonstrated to play a significant role in the control of smooth muscle phenotype and in a recent report we investigated their role in vascular mechanosensing. By smooth muscle specific deletion of Dicer, we found that microRNAs are essential for smooth muscle differentiation in response to stretch by regulating CamKIIδ and L-type calcium channel expression. Furthermore, we suggest that loss of L-type calcium channels in Dicer KO is due to reduced expression of the smooth muscle-enriched microRNA, miR-145, which targets CamKIIδ. These results unveil a novel mechanism for miR-145 dependent regulation of smooth muscle phenotype.
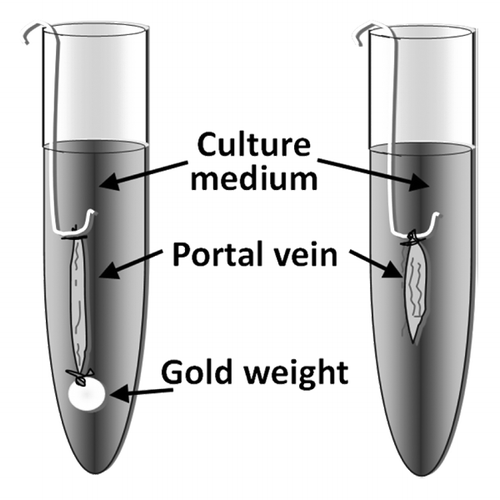
Smooth muscle cells surrounding hollow organs such as the blood vessels, the urinary bladder or the gastrointestinal tract are continuously subjected to mechanical forces. It is known that mechanical stretch can regulate smooth muscle function by stimulating intracellular signaling events, which control smooth muscle cell differentiation and growth.Citation1,Citation2 However, studies of this phenomenon in cultured cells must be interpreted with caution since cellular mechanosensing is highly dependent on the surrounding environment including cell-cell and cell-matrix interactions. In vivo, on the other hand, the effects of mechanical stretch in the vasculature can be difficult to separate from compensatory mechanisms regulating blood pressure and blood flow in the body. We have in several studies used the murine portal vein in organ culture as a model for examining stretch dependent effects in vascular smooth muscle.Citation3-Citation7 Similar to certain small arteries, the portal vein smooth muscle exhibits myogenic tone and phasic activity, which may be important factors for stretch-induced effects. Since the portal vein consists of mostly longitudinal smooth muscle, stretch is applied by attaching a weight, which corresponds to the optimal load for force development at one end of the vessel (). The vessels are then incubated in an organ culture environment for up to 5 days. Mechanical stretch of the portal vein results in an early activation of the MAPK/ERK pathway and smooth muscle growth.Citation4,Citation6,Citation7 This is followed by a delayed activation of the Rho/Rho-kinase/cofilin pathway, which promotes actin polymerization.Citation5 When actin is polymerized, it releases the transcription factor myocardin related transcription factor (MRTF) for nuclear translocation.Citation8 Like myocardin, MRTF is a co-factor for the transcription factor serum response factor (SRF), which promotes the transcription of smooth muscle specific genes. The concerted action of MRTF and SRF thereby increases contractile differentiation of smooth muscle cells. The identity of the sensor that activates signaling pathways in response to stretch is still unclear but it is likely that stretch sensitive components in the plasma membrane such as stretch-sensitive ion channels, G-protein coupled receptors or integrins coupled to focal adhesion kinase play an important role.Citation9-Citation11 Interestingly, activation of focal adhesion kinase is biphasic in response to stretch in the portal vein with an early peak that correlates with MAPK activation and a delayed peak, which correlates with Rho activation.Citation4 It is thus possible that integrins and focal adhesions are involved in stretch-induced activation of both pathways. Earlier studies have shown that L-type calcium channel influx is an important mediator for activation of the Rho pathway.Citation12,Citation13 Using pharmacological calcium channel inhibitors we found that stretch-induced MAPK pathway activation in the portal vein depends on store-operated calcium influx while Rho pathway activation requires L-type calcium channel activation.Citation14 This suggests that these signaling pathways may be selectively activated depending on the mode of influx and/or intracellular release of calcium.
Figure 1. Mouse portal veins are stretched by attaching a gold weight at one end of the vessel. The portal veins are then placed in a cell culture incubator for up to 5 days.
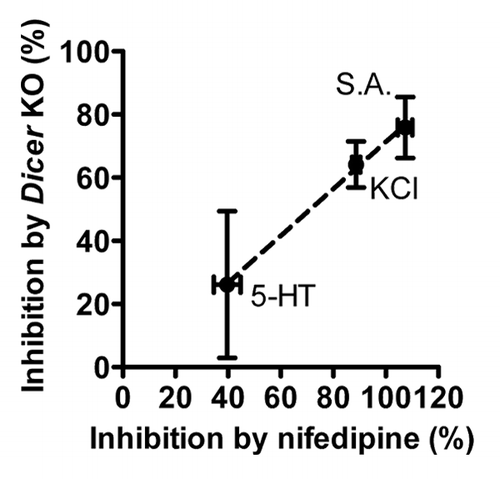
A novel mechanism involved in the regulation of protein expression and cell function was revealed by the discovery of microRNAs (miRNAs).Citation15 These small noncoding RNAs bind the 3′UTR of their target mRNA, which in most cases results in inhibition of protein translation or degradation of the mRNA. MicroRNAs are known to play an important role in smooth muscle development and contractile differentiation and a number of specific miRNAs have been identified to be of particular importance.Citation16-Citation24 In a recent study, we examined the role of miRNAs in smooth muscle mechanosensing in portal vein by using tamoxifen-inducible and smooth muscle specific Dicer KO mice (Dicer KO).Citation25 Dicer is an essential enzyme for the biosynthesis of most miRNAs and deletion of Dicer therefore results in a general loss of miRNA expression. Similar to other vascular beds, Dicer KO portal veins exhibited a reduced basal expression of smooth muscle contractile markers, confirming the important role of miRNAs in smooth muscle differentiation. Interestingly, we found that stretch-induced expression of smooth muscle markers was reduced or ablated in Dicer KO portal veins suggesting that miRNA expression is essential for stretch-induced contractile differentiation. However, it is important to note that mechanosensing per se was not affected by loss of miRNAs since acute stretch-induced MAPK activation was maintained in Dicer KO vessels. It is thus likely that the effect of Dicer KO on stretch-induced contractile differentiation is downstream of activation of integrins and focal adhesions. The Dicer KO portal veins exhibited a reduced expression of the pore forming α1C subunit of voltage dependent L-type calcium channels at both mRNA (Cacna1c) and protein (Cav1.2) levels. As mentioned earlier, inhibition of L-type calcium channels using verapamil or nifedipine is sufficient to prevent stretch-induced contractile differentiation.Citation14 A key finding of our recent study was a close correlation between effects on force of Ca2+-channel inhibition and Dicer deletion, respectively. This suggests that Dicer deletion impairs force in smooth muscle in part via effects on L-type Ca2+ channels. The observed correlation is illustrated in , where the effects of Dicer KO on responses to contractile agonists are seen to correlate with the effects of nifedipine in wild type vessels. The reduced Cacna1c expression in Dicer KO portal veins suggests a transcriptional effect on the L-type calcium channels medicated by miRNAs. Since miRNAs generally repress protein translation of their target we hypothesized that L-type calcium channels could be indirectly regulated in Dicer KO portal veins by a transcription factor or signaling molecule that inhibits L-type calcium channel expression and is upregulated in the absence of miRNAs. In a recent study by Ronkainen et al. CamKIIδ was shown to inhibit L-type calcium channel expression via the transcriptional inhibitor calsenilin/DREAM/KChIP3.Citation26 Furthermore, CamKII KO mice display an increased expression of L-type calcium channels in cardiomyocytes.Citation27 In addition to DREAM translocation, the effect of CamKII KO may depend on decreased nuclear translocation of the NFκB component p65, which suppresses transcription of Cacna1c.Citation27 Since CamKIIδ is a confirmed target of miR-145 in vascular smooth muscle cellsCitation17 and is upregulated in Dicer KO portal veins,Citation25 we hypothesized that miR-145 could regulate L-type calcium channels via CamKII. To test this hypothesis, we used isolated smooth muscle cells in culture transfected with miR-145 inhibitor. Interestingly, inhibition of miR-145 resulted in a reduction of L-type calcium channel mRNA expression, which closely correlated with the effect observed in Dicer KO portal vein. This indicates that the reduced expression of L-type calcium channel in Dicer KO smooth muscle is primarily caused by loss of miR-145. Furthermore, the effect of miR-145 on L-type calcium channel expression was prevented by the CamKII inhibitor, KN93.
Figure 2. The level of inhibition of various contractile responses in portal vein by Dicer KO correlates with force inhibition by the L-type calcium channel blocker Nifedipine. 5-HT: serotonin, KCl: potassium chloride, S.A.: Spontaneous activity. n = 3–4.
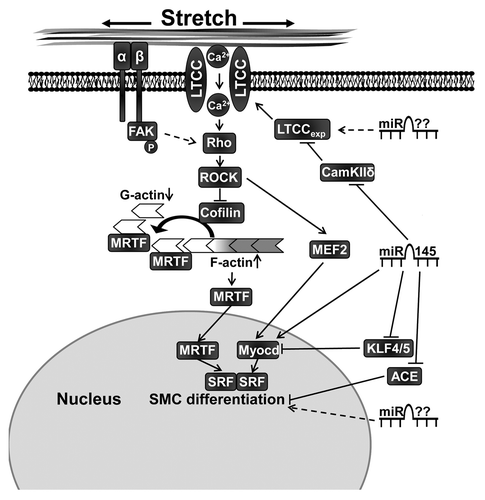
MiR-145 has previously been demonstrated to promote smooth muscle differentiation by targeting multiple factors involved in the regulation of smooth muscle phenotype including Krüppel-like factors,Citation17,Citation19 myocardinCitation17 and angiotensin converting enzyme.Citation16 Regulation of the L-type calcium channel by miR-145 therefore represents an additional mechanism by which miRNAs can control smooth muscle differentiation and contractile function (). We and others have shown that miR-145 is involved in smooth muscle actin polymerization but the role of L-type calcium influx in this process is not fully understood.Citation18,Citation22 Although miR-145 has been shown to directly target several factors involved in actin dynamics it is tempting to speculate that miR-145 promotes actin polymerization via increased expression of L-type calcium channels.
Figure 3. Contractile differentiation of vascular smooth muscle cells is promoted by mechanical stretch and miR-145. Regulation of L-type calcium expression (LTCCexp) via miR-145 and possibly other miRNAs plays an important role for stretch-induced differentiation. Stretch activates the Rho/Rho-kinase (ROCK), which promotes actin polymerization partly via inhibition of cofilin. Myocardin related transcription factor (MRTF) is then released from monomeric actin (G-actin) and translocates to the nucleus where it, as a co-factor to serum response factor (SRF), promotes smooth muscle differentiation. MicroRNA-145 also regulates contractile differentiation via additional targets such as angiotensin converting enzyme (ACE), Kruppel-like transcription factors (KLF) 4 and 5 and a direct positive regulation of myocardin (Myocd). Furthermore, it is likely that several so far unknown miRNAs are involved in smooth muscle cell (SMC) contractile differentiation. FAK, focal adhesion kinase; MEF2, myocyte enhancer factor-2.
Although we find the portal vein to be a robust model to investigate stretch-induced effects in vascular smooth muscle, the effects of Dicer KO may differ between veins and arteries and even between different arterial beds. It is therefore important to investigate the role of miRNAs in pressure-induced effects in small resistance arteries as well. Regulation of L-type calcium channel expression by miRNAs, may play a role in other cell types including cardiomyocytes and skeletal muscle. Indeed, we recently reported that smooth muscle specific deletion of Dicer also results in reduced expression of L-type calcium channels in smooth muscle of the urinary bladder, indicating that miRNAs regulate these channels in multiple tissues.Citation28
Disclosure of Potential Conflicts of Interest
There were no potential conflicts of interest to disclose.
Acknowledgments
This work was supported in part by the Swedish Research Council; the Swedish Heart and Lung Foundation; The Crafoord Foundation; The Royal Physiographic Society; The Ake Wiberg Foundation; The Tore Nilson Foundation; The Greta and Johan Kock Foundation; The Magnus Bergvall Foundation and The Lars Hierta Memorial Foundation.
References
- Hellstrand P, Albinsson S. Stretch-dependent growth and differentiation in vascular smooth muscle: role of the actin cytoskeleton. Can J Physiol Pharmacol 2005; 83:869 - 75; http://dx.doi.org/10.1139/y05-061; PMID: 16333359
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 2004; 84:767 - 801; http://dx.doi.org/10.1152/physrev.00041.2003; PMID: 15269336
- Albinsson S, Nordström I, Swärd K, Hellstrand P. Differential dependence of stretch and shear stress signaling on caveolin-1 in the vascular wall. Am J Physiol Cell Physiol 2008; 294:C271 - 9; http://dx.doi.org/10.1152/ajpcell.00297.2007; PMID: 17989209
- Albinsson S, Hellstrand P. Integration of signal pathways for stretch-dependent growth and differentiation in vascular smooth muscle. Am J Physiol Cell Physiol 2007; 293:C772 - 82; http://dx.doi.org/10.1152/ajpcell.00622.2006; PMID: 17507430
- Albinsson S, Nordström I, Hellstrand P. Stretch of the vascular wall induces smooth muscle differentiation by promoting actin polymerization. J Biol Chem 2004; 279:34849 - 55; http://dx.doi.org/10.1074/jbc.M403370200; PMID: 15184395
- Zeidan A, Nordström I, Albinsson S, Malmqvist U, Swärd K, Hellstrand P. Stretch-induced contractile differentiation of vascular smooth muscle: sensitivity to actin polymerization inhibitors. Am J Physiol Cell Physiol 2003; 284:C1387 - 96; PMID: 12734104
- Zeidan A, Nordström I, Dreja K, Malmqvist U, Hellstrand P. Stretch-dependent modulation of contractility and growth in smooth muscle of rat portal vein. Circ Res 2000; 87:228 - 34; http://dx.doi.org/10.1161/01.RES.87.3.228; PMID: 10926874
- Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell 2003; 113:329 - 42; http://dx.doi.org/10.1016/S0092-8674(03)00278-2; PMID: 12732141
- Langton PD. Calcium channel currents recorded from isolated myocytes of rat basilar artery are stretch sensitive. J Physiol 1993; 471:1 - 11; PMID: 8120799
- Mederos y Schnitzler M, Storch U, Gudermann T. AT1 receptors as mechanosensors. Curr Opin Pharmacol 2011; 11:112 - 6; http://dx.doi.org/10.1016/j.coph.2010.11.003; PMID: 21147033
- Lehoux S, Esposito B, Merval R, Tedgui A. Differential regulation of vascular focal adhesion kinase by steady stretch and pulsatility. Circulation 2005; 111:643 - 9; http://dx.doi.org/10.1161/01.CIR.0000154548.16191.2F; PMID: 15668343
- Wamhoff BR, Bowles DK, McDonald OG, Sinha S, Somlyo AP, Somlyo AV, et al. L-type voltage-gated Ca2+ channels modulate expression of smooth muscle differentiation marker genes via a rho kinase/myocardin/SRF-dependent mechanism. Circ Res 2004; 95:406 - 14; http://dx.doi.org/10.1161/01.RES.0000138582.36921.9e; PMID: 15256479
- Ratz PH, Berg KM, Urban NH, Miner AS. Regulation of smooth muscle calcium sensitivity: KCl as a calcium-sensitizing stimulus. Am J Physiol Cell Physiol 2005; 288:C769 - 83; http://dx.doi.org/10.1152/ajpcell.00529.2004; PMID: 15761211
- Ren J, Albinsson S, Hellstrand P. Distinct effects of voltage- and store-dependent calcium influx on stretch-induced differentiation and growth in vascular smooth muscle. J Biol Chem 2010; 285:31829 - 39; http://dx.doi.org/10.1074/jbc.M109.097576; PMID: 20675376
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116:281 - 97; http://dx.doi.org/10.1016/S0092-8674(04)00045-5; PMID: 14744438
- Boettger T, Beetz N, Kostin S, Schneider J, Krüger M, Hein L, et al. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J Clin Invest 2009; 119:2634 - 47; http://dx.doi.org/10.1172/JCI38864; PMID: 19690389
- Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, et al. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 2009; 460:705 - 10; PMID: 19578358
- Xin M, Small EM, Sutherland LB, Qi X, McAnally J, Plato CF, et al. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev 2009; 23:2166 - 78; http://dx.doi.org/10.1101/gad.1842409; PMID: 19720868
- Cheng Y, Liu X, Yang J, Lin Y, Xu DZ, Lu Q, et al. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ Res 2009; 105:158 - 66; http://dx.doi.org/10.1161/CIRCRESAHA.109.197517; PMID: 19542014
- Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, et al. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res 2007; 100:1579 - 88; http://dx.doi.org/10.1161/CIRCRESAHA.106.141986; PMID: 17478730
- Liu X, Cheng Y, Zhang S, Lin Y, Yang J, Zhang C. A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res 2009; 104:476 - 87; http://dx.doi.org/10.1161/CIRCRESAHA.108.185363; PMID: 19150885
- Albinsson S, Suarez Y, Skoura A, Offermanns S, Miano JM, Sessa WC. MicroRNAs are necessary for vascular smooth muscle growth, differentiation, and function. Arterioscler Thromb Vasc Biol 2010; 30:1118 - 26; http://dx.doi.org/10.1161/ATVBAHA.109.200873; PMID: 20378849
- Albinsson S, Sessa WC. Can microRNAs control vascular smooth muscle phenotypic modulation and the response to injury?. Physiol Genomics 2011; 43:529 - 33; http://dx.doi.org/10.1152/physiolgenomics.00146.2010; PMID: 20841497
- Albinsson S, Skoura A, Yu J, DiLorenzo A, Fernández-Hernando C, Offermanns S, et al. Smooth muscle miRNAs are critical for post-natal regulation of blood pressure and vascular function. PLoS ONE 2011; 6:e18869; http://dx.doi.org/10.1371/journal.pone.0018869; PMID: 21526127
- Turczynska KM, Sadegh MK, Hellstrand P, Swärd K, Albinsson S. MicroRNAs are essential for stretch-induced vascular smooth muscle contractile differentiation via microRNA (miR)-145-dependent expression of L-type calcium channels. J Biol Chem 2012; 287:19199 - 206; http://dx.doi.org/10.1074/jbc.M112.341073; PMID: 22474293
- Ronkainen JJ, Hänninen SL, Korhonen T, Koivumäki JT, Skoumal R, Rautio S, et al. Ca2+-calmodulin-dependent protein kinase II represses cardiac transcription of the L-type calcium channel alpha(1C)-subunit gene (Cacna1c) by DREAM translocation. J Physiol 2011; 589:2669 - 86; http://dx.doi.org/10.1113/jphysiol.2010.201400; PMID: 21486818
- Xu L, Lai D, Cheng J, Lim HJ, Keskanokwong T, Backs J, et al. Alterations of L-type calcium current and cardiac function in CaMKIIdelta knockout mice. Circ Res 2010; 107:398 - 407; http://dx.doi.org/10.1161/CIRCRESAHA.110.222562; PMID: 20538682
- Sadegh MK, Ekman M, Rippe C, Uvelius B, Swärd K, Albinsson S. Deletion of Dicer in smooth muscle affects voiding pattern and reduces detrusor contractility and neuroeffector transmission. PLoS ONE 2012; 7:e35882; http://dx.doi.org/10.1371/journal.pone.0035882; PMID: 22558254