Abstract
Jetlag results from the misalignment between the endogenous circadian timing and the civil timing after a transmeridian flight. Efficacy of the dim nocturnal illumination (0.03 lx) in accelerating the reentrainment following simulated jetlags in Drosophila biarmipes was examined by subjecting the flies to 24 h light-dark cycles in which the 12 h photophase was at 300 lx for all flies but the scotophase was at 0 and 0.03 lx for the control and experimental flies, respectively. Reentrainment was always faster in the experimental flies than the control ones. Moreover, unlike melatonin, the dimly lit nights accelerated the reentrainment following both, the phase advance and delay of the light-dark cycles. This study might have potential application as a non-drug jetlag treatment.
Introduction
Entrainment in nature is accomplished by exposure of animals to the cycles of day and night, which consist of three powerful photic cues: the daylight, twilight transitions and the natural dim scotopic illumination.Citation1,Citation2 Although the dim illumination failed to evoke any phase shifts or suppress the melatonin secretion in nocturnal rodents,Citation3 it was regarded as a persuasive photic contributor capable of altering the basic properties of the circadian pacemaker.Citation4 For example, the dimly lit nights modified the waveform of activity rhythms of the white-footed mice, bats and owl-monkeys.Citation5-Citation7 Dim nocturnal illumination in hamsters extended the range of entrainment, increased the incident of dissociation of locomotor activity rhythm and showed the phase-dependent effects on reentrainment and masking.Citation4,Citation8-Citation11 Moreover, the continuous dim green light in hamsters lengthened the period of free-running rhythm and increased the duration of activity by ~3 h when compared with the hamsters in continuous complete darkness.Citation12 The dim nocturnal irradiance of ~0.2 lx was reported to accelerate the reentrainment in the young and old hamsters following simulated advance and delay jetlags of 4 and 8 h.Citation13
Dimly-lit nights modified the basic properties of the pacemakers controlling the pupal eclosion and adult locomotor activity rhythms of a few insects too. For example, the dim natural and artificial nighttime illuminations altered the attributes of entrainment and free-running rhythm of the pupal eclosion of D. jambulina.Citation14 In the same species, the nocturnal illumination at 0.0006 lx advanced the activity onset by several hours, extended the activity duration, enhanced the activity level and shortened the period of free-running rhythm.Citation15 Varying durations (9–15 h) of the dim scotophase at 0.0006 lx modulated the fundamental properties of the circadian pacemakers that controlled the adult locomotor activity rhythm of D. jambulina.Citation2 Dim nocturnal irradiance in D. melanogaster also advanced the morning peak but delayed the evening peak of activity that rendered the flies completely nocturnalCitation16,Citation17 Moreover, it also altered the clock protein rhythms in the pacemaker neurons of the brain. Dim illumination even during the daytime had profound influence on the locomotor activity rhythms of a few insects. For instance, the dim photophase at 1 lx delayed the onset of the locomotor activity rhythm of the onion fly, Delia antiqua and lengthened the period of free-running rhythm in continuous dim light.Citation18 The dim photophase contrary to the bright one, enhanced the activity level and stimulated the behavioral components like the resting, grooming and feeding in D. melanogaster.Citation19
Thus, these studies revealed that the dim naturalistic light during the night or even during the day had multitude of effects on the circadian features of several mammals and insects. Nevertheless, the influence of the dimly lit nights in accelerating the reentrainment following simulated jetlags has not been reported yet in any diurnal animal model. The objective of the present study was to examine the efficacy of the dimly lit nights in accelerating the reentrainment of the circadian rhythm of the adult locomotor activity of Drosophila biarmipes, which is predominantly a diurnal insect in nature. This report is the part of ongoing investigation in the ability of the photic zeitgebers to influence the reentrainment following the simulated jetlags in D. biarmipes. Recently, the authors have demonstrated that the bright photophase at 300 lx as compared with the dim photophase at 30 lx, speeded up the reentrainment in this species following the phase advance and delay of light-dark cycles.Citation20 Even the duration of photophase influenced the rate of reentrainment in D. biarmipes, however, it was dependent on the direction of the shifts in light-dark cycles. The short photophase of 9 h and the long photophase of 15 h accelerated the reentrainment following simulated advance and delay jetlags, respectively.Citation21
Methods
The stock culture of the wild-type strain designated as MD-101 of Drosophila biarmipes was maintained at 21° ± 0.5°C and ~60% relative humidity (r.h.) in light-dark (LD) cycles of 12 h of white light at 300 lx and 12 h of complete darkness which are regarded as the standard LD cycles. The broad spectrum (365–810 nm) white light obtained from the UV-free white light emitting diodes (6 VDC-0.02W, the angle of light emission 20–30°, Ligitek Electronics Co.) was used during the photophase at 300 lx as well as during the dim scotophase at 0.03 lx. The computerized photoelectric method for recording the adult locomotor activity rhythm of Drosophila has been described in details.Citation2,Citation15,Citation22 However, the method in brief is as follows. Three units of the Chronobiology Kit (Stanford Software Systems, Release Version 1c, 1998–2004-62-channel) were used for recording the activity rhythm and for the analysis of the circadian parameters. One-day old males were individually introduced in an activity recording glass tube (100 mm long × 7 mm outer diameter). One end of the tube was inserted in 5 g of culture medium kept in a plastic container (vol. 10 ml) which served as the source of food and water for the fly. The other end of the tube was closed with a loose cotton-plug for aeration. This arrangement allowed the uninterrupted recording of the activity for ~10 d. Thereafter, the fly was transferred to a fresh tube to continue the data recording. Each tube was placed in the path of an infrared beam (peak transmission λ of 738 nm) of an activity recording device. Twenty such activity recording devices were housed in each of 35 light-proof wooden-enclosures (60 × 80 × 90 cm) kept in a room maintained at 21° ± 0.5°C and ~60% r.h.
Efficacy of the dim nocturnal illumination in accelerating the reentrainment was examined by subjecting the flies to two types of lighting schedules wherein the 12 h photophase was at 300 lx for all flies but the 12 h scotophase was at 0 l× (D) for the control flies and 0.03 lx, i.e., the artificial starlight (S) for the experimental flies. Flies were maintained for two generations in each lighting schedule to avoid the after-effects of the standard LD cycles in which they were bred for several generations. Two experiments were performed on the one-day old males (n = 49) of the third generation in the same lighting regime in which they were maintained.
The first experiment examined whether the synchronization of the control and experimental flies by LD 12:12 and LS 12:12 cycles, respectively, was the circadian entrainment or the masking effects of the scotopic illumination. This was accomplished by subjecting the flies to each lighting schedule for 10 d and thereafter, transferring them to continuous darkness (DD) that commenced at the lights-on phase of the day 11. Five parameters of entrainment were evaluated: the phase-angle difference between the activity onset (Aon) phase of the morning (M) peak and the lights-on transition (ψo), the phase-angle difference between the activity offset (Aoff) phase of the evening (E) peak and the lights-off transition (ψe), the phase-relationship between the Aoff phase of the M peak and the Aon phase of the E peak (ψM-E), the duration of activity (α), and the duration of the rest (ρ). The mean activity profile during entrainment of 10 d in each lighting regime was presented in 0.25 h bins (mean ± SD, n = 49 flies) to distinguish the circadian peak (the expected peak appearing before the lights-on or lights-off transition) from the masking peak (the short activity burst in response to the lights-on or lights-off transition). The entrainment was comprehensively determined by considering the three forms of stable phase relationships (the ψo, ψM-E and ψe) in each lighting schedule and the predictable phase (the Aon phase of the M peak of the last day of the entrainment) from which the free-run commenced upon transfer to DD. Activity level (AL) was defined as the average number of activity passes per fly per day from the pooled data of 49 flies during the entrainment and the stable free-running state in DD. The period of free-running rhythm (τ) in DD was computed by the chi-square periodogram analysis (step-size = 5 min).
The second experiment examined the efficacy of the dim nocturnal irradiance in influencing the reentrainment. Initially, the control and experimental flies were entrained for 10 d by LD 12:12 and LS 12:12 cycles, respectively. On day 11, the lighting regime was abruptly phase advanced or phase delayed by shortening and lengthening of the scotophase by 8 h, respectively. Each shifted lighting schedule was continued for 18–23 d until the stable reentrainment was instituted. It took several days for the flies to re-entrain and the reentrainment was regarded to have accomplished when the three forms of the previous phase relationships (the ψo, ψM-E and the ψe) were reestablished in the shifted lighting schedule. Transients were the temporary oscillatory states of the Aon and Aoff phases between the initial entrainment and the subsequent stable reentrainment. Advancing and/or delaying transients endured by the Aon and Aoff phases were individually counted to distinguish the different rate of reentrainment of these two phase markers. There was always a single activity bout during 1–4 transients immediately following the advance or delay shift in the lighting schedule, therefore the offset of such single activity bout was regarded as the Aoff phase of the prospective E peak of the rhythm. Furthermore, two forms of transients were distinguished: the orthodromic form in which the transients were in a direction of the shifted LD cycle, and the antidromic form in which the transients were in a direction opposite to the shifted LD cycle.Citation23 Double plotted actograms were presented in a percentile distribution format. Values of the parameters of entrainment and free-running rhythmicity were the means (± SD, n = 49 flies). The Wilcoxon-Mann-Whitney test was performed on the activity data of both experiments to see any significant effect of the dim scotopic irradiance.
Results
The show the actograms of the representative control and experimental flies of D. biarmipes entrained by the LD 12:12 and LS 12:12 cycles, respectively. All flies exhibited the bimodal activity pattern with the major M peak and the minor E peak as shown in their activity profiles (). The control flies were characterized by the circadian M and E peaks as well as the masking peaks at the lights-on and lights-off transitions (). The experimental flies too showed the circadian M and E peaks, in addition to the lights-on induced masking peak, nonetheless, the lights-off induced masking peak was perpetually missing (). Moreover, the Aoff phase of the E peak always occurred just before the lights-off transitions since the activity was never extended in the early part of the dimly lit night. After-effects of the nocturnal illumination on τ were examined by transferring the flies from each lighting regime to DD that initiated the robust free-running rhythmicity following 2.4 ± 0.8 delaying and 4.4 ± 1.1 advancing transients in the control and experimental flies, respectively (n = 49 flies in each lighting schedule). The bimodal activity pattern was never observed in any group of the flies once transferred to DD. The free-running rhythmicity was initiated from the Aon phase of the last M peak of the entrained rhythm and not from the lights-on phase of the lighting schedule. Moreover, the free-running rhythmicity was generated by the participation of the M peak only, as the E peak was clearly left out. All parameters of the entrainment and free-running rhythmicity of the experimental flies were significantly different from that of the control flies (p < 0.001) ().
Figure 1. Entrainment and free-run in D. biarmipes. The actograms of the representative control and experimental males of D. biarmipes which were entrained by LD 12:12 (A) and LS 12:12 (C) cycles, respectively, and then transferred to DD on the day 11 (oblique arrow). The mean activity profiles of 49 control (B) and experimental (D) males during the entrainment are showing the circadian peaks (dark arrows), the masking peaks (open arrows) and the phase relationship between the Aoff of the M peak and Aon of the E peak (ΨM-E). The dark and open time bars denote the photophase (300 lx) and scotophase (0 lx), respectively, the long dark time bars denote DD and the hatched portion of the time-bars denotes the dim nocturnal irradiance at 0.03 lx.
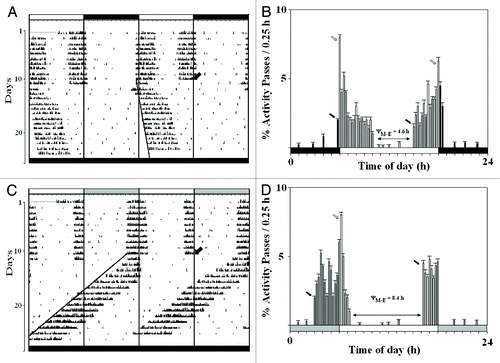
Table 1. Effects of the lighting schedules with dark nights (Control Expt.) and dimly-lit nights (LAN Expt.) on the features of entrainment and free-running rhythmicity of D. biarmipes.
The reentrainment in both groups of the flies following the phase advance as well as phase delay of the lighting schedules was also mediated by the M peak only as the E peak was never involved (). The actograms of the representative control and experimental flies following the phase advance of 8 h are presented in the , respectively. The reentrainment of the Aon and Aoff phases of both groups of the flies differed with respect to the direction and number of transients (). In both groups of the flies, the Aon phase reentrained consistently by the orthodromic advancing transients, whereas the Aoff phase initially endured the orthodromic advancing transients, and then the antidromic delaying transients () during which the α was decompressed ().
Figure 2. Simulated advance and delay jetlags in D. biarmipes. The actograms of the four representative males of D. biarmipes which were subjected to 8 h phase advance and delay of LD 12:12 cycles (A and C, respectively) and LS 12:12 cycles (B and D, respectively).
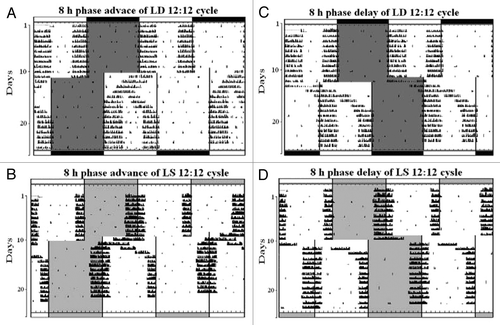
Figure 3. Transients after advance and delay jetlags. Advancing and delaying transients (mean ± S.D., n = 49 flies) endured by the activity onsets (Aon-LD) and offsets (Aoff-LD) of the control males and that of the experimental males (Aon-LS and Aoff-LS) of D. biarmipes following the advance (A) and delay shift (B) in the lighting schedules. The asterisks denote the statistically significant effects of the dim nocturnal illumination (p < 0.001).
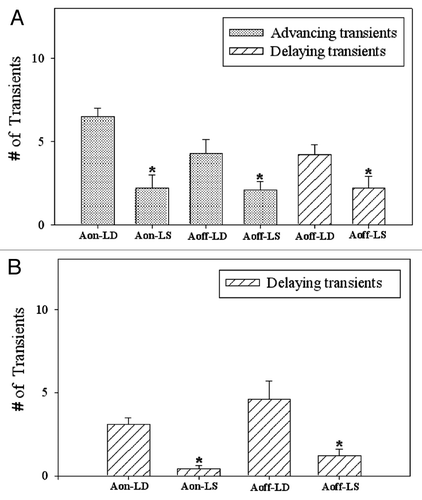
The actograms of the representative control and experimental flies after the phase delay of 8 h are present in the , respectively. Both, the Aon and Aoff phases of the control and experimental flies reentrained consistently by the orthodromic delaying transients (). The decompression of α in all flies occurred when the morning activity bout was dissociated into two components. The first component reestablished its customary phase relationship with the lights-on phase, whereas the second component was delayed until it achieved the usual phase position with the lights-off event (−D). The transients experienced by the Aon and Aoff phases of the experimental flies after the advance as well as the delay shifts in the lighting schedules were significantly less than that of the control flies (p < 0.001) (). Moreover, the total number of transients endured by the Aon phases of all flies of each group were always significantly less than the total number of transients endured by the Aoff phases after the advance or delay shift in the lighting regime (p < 0.001).
Discussion
The synchronization of the activity rhythm of the control and experimental flies of D. biarmipes by the 24 h lighting schedules with completely dark nights () and dimly-lit nights, respectively (), was the circadian entrainment indeed and not the masking effects of the scotopic lighting conditions.Citation24,Citation25 This could be corroborated by the distinctive ψo and ψe in each lighting regime, the origin of the free-running rhythmicity from the Aon phase of the rhythm and not from the lights-on phase of the light-dark cycles, the undeniable presence of the transients following the transfer from the lighting regime to DD, and the characteristic τ in DD as the after-effect of the different photic ambience of the prior scotophase. The authors have also demonstrated such influence of the extremely dim nocturnal illumination at 0.0006 lx on the attributes of entrainment and the free-running rhythmicity of the locomotor activity of D. jambulina.Citation15 Although such nocturnal illumination was 50-times dimmer than that used in the present study, it advanced the activity onset in the LS 12:12 cycles and shortened the τ in subsequent DD. These results were attributed to the very low photic sensitivity of D. jambulina. It would be interesting to examine the efficacy of such extremely dim nocturnal illumination in influencing the process of reentrainment in D. biarmipes, D. jambulina or a diurnal mammalian experimental model like the Indian palm-squirrel.
The present study comprehensively assessed the reentrainment by using five basic but often overlooked components of the entrainment: the Aon and Aoff phases as the rhythm markers, the ψM-E, α and ρ. Except for the two studies on reentrainment,Citation26,Citation27 the activity onset phase had been used as a sole rhythm marker to assess the reentrainment of locomotor activity rhythm, however, this approach fell short of projecting the accurate behavior of the underlying pacemakers during the reentrainment process.Citation28,Citation29 These studies apparently overlooked the complex organization of the waveform of the locomotor activity rhythm with five indispensable components, which are prone to be modified by various features of the lighting regime. If the Aon phase is regarded as the exclusive phase maker in the present study, then the reentrainment would be much faster than demonstrated here ( and ).
Melatonin ameliorates the jetlag-symptoms only after the phase advance of the LD cycle,Citation30 whereas, the dim nocturnal illumination indisputably accelerates the reentrainment in D. biarmipes following both, the phase advance and delay of the lighting schedules (). These results are in agreement with the acceleration of reentrainment in hamsters by the dim nocturnal irradiance.Citation13 The possible mechanism for the acceleration of reentrainment in D. biarmipes could be the photic or non-photic. The photic mechanism envisaged the direct effect of the dimly lit nights on the pacemakers. Dim scotopic illumination is documented to alter the circadian waveform of the activity rhythm by modulating the α and thereby changing the coupling mechanism between the M and E oscillators.Citation8,Citation12 The dim nighttime illumination unequivocally altered the waveform of the activity rhythm of D. biarmipes as the α of the experimental flies during both, the entrained and free-running states was considerably longer than that of the control flies (). Such change in the waveform of the activity rhythm apparently augmented the flexibility of the underlying pacemakers resulting in minimizing the desynchrony among the constituent oscillators following the abrupt shifts in the LS cycle. That obviously reduced the number of transients endured by the Aon and Aoff phases of the experimental flies. The existence of such a desynchrony among molecular components of the pacemakers controlling the locomotor activity rhythm of rodents was shown to prolong the reentrainment.Citation29,Citation31
The non-photic mechanism for accelerating the reentrainment in D. biarmipes would involve the indirect effects of the dim nights on the pacemakers. A remarkable increment in the activity level caused by the dimly lit nights might act as the potential non-photic input to the pacemaker. Such a non-photic input—although not induced by the dim light—speeded up the resynchronization in rodents too. For instance, the activity increment immediately following the phase advance of the LD cycles resulted in an impressive acceleration of reentrainment in the hamsters.Citation32 An increment in the activity level caused by the dim scotopic irradiance was demonstrated to act through a non-photic mechanism too that influenced the entrainment and phase resetting in hamsters. The dim scotopic illumination apparently promoted the dissociation of the activity rhythm by facilitating the non-photic mechanism as well as the photic mechanism in the hamsters subjected to the LDLD 7:5:7:5 cycles.Citation8 Furthermore, the dim nocturnal irradiance influenced the reentrainment in the hamsters by altering the magnitude of the phase resetting caused either by the bright light or the non-photic input such as the cage-changes.Citation12 The dim nocturnal illumination also augmented the activity level of the young hamsters but the possibility of its role as a non-photic zeitgeber to speed up the resynchronization was ruled out.Citation13 Since the old hamsters exposed to the dimly-lit nights reentrained always faster than those exposed to the dark-nights, despite the both groups of hamsters had a comparable activity level. That prompted the authors to investigate the influence of the activity increment caused by the physical exercise scheduleCitation33 on the rate of reentrainment in D. biarmipes (Sinam et al., unpublished data). An impressive increment of about 29% in the activity level was observed in the dark-night exposed males (n = 69) of D. biarmipes when subjected to 1 h bout of physical exercise immediately following the 6 h advance shift in the LD cycles. However, such increment in the activity level failed to accelerate the reentrainment. Therefore, the effectiveness of the activity increment induced by the dim-nights as a non-photic input to the pacemakers to accelerate the reentrainment in D. biarmipes should be negated in the present study.
Other features of the photic zeitgeber were documented to accelerate the reentrainment of D. biarmipes, too. For instance, the bright photophase akin to the dim nocturnal illumination in the present study, indiscriminately accelerated the reentrainment in D. biarmipes, following shifts in LD cycles in both the directions.Citation20 These results were ascribed to the enhanced zeitgeber strength of the bright photophase that reinforced the coupling mechanism of the Aon and Aoff phases with the lights-on and lights-off transitions, respectively. The duration of photophase also influenced the reentrainment in this species, nevertheless, it depended on the direction of the LD cycle shift.Citation21 The short photophase accelerated the reentrainment only after the phase advance, whereas the long photophase accelerated the reentrainment only after the phase delay of the LD cycles. These results were attributed to the early Aon phase and short τ of the flies exposed to the short photophase, and the opposite was true for the flies exposed to the long photophase. Such studies may have the practical application as a non-drug treatment to minimize the jetlag problems, especially, among the frequent flyers like the cabin-crew, sportspersons, etc., who are legally prohibited from taking certain pharmaceutical preparations for jetlags.
| Abbreviations: | ||
| AL | = | activity level |
| α | = | duration of activity |
| Aon | = | activity onset |
| Aoff | = | activity offset |
| DD | = | continuous darkness |
| M | = | morning |
| E | = | evening |
| S | = | artificial starlight |
| LD | = | light-dark |
| Ψ | = | phase angle difference |
| ΨM-E | = | phase relationship between the morning and evening oscillators |
| τ | = | period of free-running rhythm |
Acknowledgments
The study benefitted from the equipment obtained under the Research Grant No. FIST SR/SO/AS-MS01/2009 funded by Department of Science and Technology, New Delhi to D.S.J. The authors thank two anonymous reviewers for their constructive comments on the previous version of the manuscript.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Roenneberg T, Foster RG. Twilight times: light and the circadian system. Photochem Photobiol 1997; 66:549 - 61; http://dx.doi.org/10.1111/j.1751-1097.1997.tb03188.x; PMID: 9383985
- Thakurdas P, Sharma S, Singh B, Vanlalhriatpuia K, Joshi D. Varying the length of dim nocturnal illumination differentially affects the pacemaker controlling the locomotor activity rhythm of Drosophila jambulina.. Chronobiol Int 2011; 28:390 - 6; http://dx.doi.org/10.3109/07420528.2011.574021; PMID: 21721854
- Nelson DE, Takahashi JS. Comparison of visual sensitivity for suppression of pineal melatonin and circadian phase-shifting in the golden hamster. Brain Res 1991; 554:272 - 7; http://dx.doi.org/10.1016/0006-8993(91)90200-F; PMID: 1933309
- Gorman MR, Kendall ME, Elliott JA. Scotopic illumination enhances entrainment of circadian rhythms to lengthening light:dark cycles. J Biol Rhythms 2005; 20:38 - 48; http://dx.doi.org/10.1177/0748730404271573; PMID: 15654069
- Kavanau JL. Behavior of captive white-footed mice. Science 1967; 155:1623 - 39; http://dx.doi.org/10.1126/science.155.3770.1623; PMID: 6020284
- Erkert H, Bay F, Kracht S. Zeitgeber induced modulation of activity patterns in nocturnal mammals (Chiroptera). Experientia 1976; 32:560 - 2; http://dx.doi.org/10.1007/BF01990160; PMID: 1278292
- Erkert HG, Gröber J. Direct modulation of activity and body temperature of owl monkeys (Aotus lemurinus griseimembra) by low light intensities. Folia Primatol (Basel) 1986; 47:171 - 88; http://dx.doi.org/10.1159/000156276; PMID: 3609970
- Evans JA, Elliott JA, Gorman MR. Circadian entrainment and phase resetting differ markedly under dimly illuminated versus completely dark nights. Behav Brain Res 2005; 162:116 - 26; http://dx.doi.org/10.1016/j.bbr.2005.03.014; PMID: 15922072
- Gorman MR, Evans JA, Elliott JA. Potent circadian effects of dim illumination at night in hamsters. Chronobiol Int 2006; 23:245 - 50; http://dx.doi.org/10.1080/07420520500521905; PMID: 16687298
- Gorman MR, Elliott JA. Dim nocturnal illumination alters coupling of circadian pacemakers in Siberian hamsters, Phodopus sungorus.. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 2004; 190:631 - 9; http://dx.doi.org/10.1007/s00359-004-0522-7; PMID: 15127217
- Frank DW, Evans JA, Gorman MR. Time-dependent effects of dim light at night on reentrainment and masking of hamster activity rhythms. J Biol Rhythms 2010; 25:103 - 12; http://dx.doi.org/10.1177/0748730409360890; PMID: 20348461
- Evans JA, Elliott JA, Gorman MR. Circadian effects of light no brighter than moonlight. J Biol Rhythms 2007; 22:356 - 67; http://dx.doi.org/10.1177/0748730407301988; PMID: 17660452
- Evans JA, Elliott JA, Gorman MR. Dim nighttime illumination accelerates adjustment to timezone travel in an animal model. Curr Biol 2009; 19:R156 - 7; http://dx.doi.org/10.1016/j.cub.2009.01.023; PMID: 19243688
- Thakurdas P, Sharma S, Vanlalhriatpuia K, Sinam B, Chib M, Shivagaje A, et al. Light at night alters the parameters of the eclosion rhythm in a tropical fruit fly, Drosophila jambulina.. Chronobiol Int 2009; 26:1575 - 86; http://dx.doi.org/10.3109/07420520903529765; PMID: 20030541
- Thakurdas P, Sharma S, Sinam B, Chib M, Joshi D. Nocturnal illumination dimmer than starlight altered the circadian rhythm of adult locomotor activity of a fruit fly. Chronobiol Int 2010; 27:83 - 94; http://dx.doi.org/10.3109/07420520903398567; PMID: 20205559
- Bachleitner W, Kempinger L, Wülbeck C, Rieger D, Helfrich-Förster C. Moonlight shifts the endogenous clock of Drosophila melanogaster.. Proc Natl Acad Sci USA 2007; 104:3538 - 43; http://dx.doi.org/10.1073/pnas.0606870104; PMID: 17307880
- Kempinger L, Dittmann R, Rieger D, Helfrich-Forster C. The nocturnal activity of fruit flies exposed to artificial moonlight is partly caused by direct light effects on the activity level that bypass the endogenous clock. Chronobiol Int 2009; 26:151 - 66; http://dx.doi.org/10.1080/07420520902747124; PMID: 19212834
- Watari Y, Arai T. Effect of dim light on locomotor activity rhythm in the onion fly, Delia antiqua.. Zoolog Sci 1999; 16:603 - 9; http://dx.doi.org/10.2108/zsj.16.603
- Rieger D, Fraunholz C, Popp J, Bichler D, Dittmann R, Helfrich-Förster C. The fruit fly Drosophila melanogaster favors dim light and times its activity peaks to early dawn and late dusk. J Biol Rhythms 2007; 22:387 - 99; http://dx.doi.org/10.1177/0748730407306198; PMID: 17876060
- Sinam B, Sharma S, Thakurdas P, Joshi DS. Bright photophase accelerates reentrainment after experimental jetlag in Drosophila.. Naturwissenschaften 2012; 99:575 - 8; http://dx.doi.org/10.1007/s00114-012-0928-y; PMID: 22684252
- Sinam B, Sharma S, Thakurdas P, Joshi D. Influence of photoperiod in accelerating the reentrainment in Drosophila.. Chronobiol Int 2012; 29:1405 - 11
- Sharma S, Thakurdas P, Sinam B, Joshi D. Paradoxical masking effects of bright photophase and high temperature in Drosophila malerkotliana.. Chronobiol Int 2012; 29:157 - 65; http://dx.doi.org/10.3109/07420528.2011.644875; PMID: 22324554
- Nair K, Selvaraj R, Farid T, Nanthakumar K. Antidromic His capture during entrainment of orthodromic AVRT. Pacing Clin Electrophysiol 2010; 33:1153 - 6; http://dx.doi.org/10.1111/j.1540-8159.2010.02759.x; PMID: 20370852
- Daan S, Aschoff J. The entrainment of circadian systems. In: Takahashi J, Turek F, Moore R, ed(s). Handbook of behavioral neurobiology: Circadian clocks. New York: Plenum, 2001;7-43.
- Mrosovsky N. Masking: history, definitions, and measurement. Chronobiol Int 1999; 16:415 - 29; http://dx.doi.org/10.3109/07420529908998717; PMID: 10442236
- Meijer JH, De Vries MJ. Light-induced phase shifts in onset and offset of running-wheel activity in the Syrian hamster. J Biol Rhythms 1995; 10:4 - 16; http://dx.doi.org/10.1177/074873049501000101; PMID: 7632979
- Liu T, Borjigin J. Reentrainment of the circadian pacemaker through three distinct stages. J Biol Rhythms 2005; 20:441 - 50; http://dx.doi.org/10.1177/0748730405279388; PMID: 16267383
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, et al. Resetting central and peripheral circadian oscillators in transgenic rats. Science 2000; 288:682 - 5; http://dx.doi.org/10.1126/science.288.5466.682; PMID: 10784453
- Reddy AB, Field MD, Maywood ES, Hastings MH. Differential resynchronisation of circadian clock gene expression within the suprachiasmatic nuclei of mice subjected to experimental jet lag. J Neurosci 2002; 22:7326 - 30; PMID: 12196553
- Srinivasan V, Spence DW, Pandi-Perumal SR, Trakht I, Cardinali DP. Jet lag: therapeutic use of melatonin and possible application of melatonin analogs. Travel Med Infect Dis 2008; 6:17 - 28; http://dx.doi.org/10.1016/j.tmaid.2007.12.002; PMID: 18342269
- Nagano M, Adachi A, Nakahama K, Nakamura T, Tamada M, Meyer-Bernstein E, et al. An abrupt shift in the day/night cycle causes desynchrony in the mammalian circadian center. J Neurosci 2003; 23:6141 - 51; PMID: 12853433
- Mrosovsky N, Salmon PA. A behavioural method for accelerating reentrainment of rhythms to new light-dark cycles. Nature 1987; 330:372 - 3; http://dx.doi.org/10.1038/330372a0; PMID: 3683553
- Piazza N, Gosangi B, Devilla S, Arking R, Wessells R. Exercise-training in young Drosophila melanogaster reduces age-related decline in mobility and cardiac performance. PLoS ONE 2009; 4:e5886; http://dx.doi.org/10.1371/journal.pone.0005886; PMID: 19517023