Abstract
Leaf-cutting ants are well known for their highly complex social organization, which provides them with a strong defense against parasites invading their colonies. Besides this attribute, these insects have morphological, physiological and structural characteristics further reinforcing the defense of their colonies. With the discovery of symbiotic bacteria present on the integument of leaf-cutting ants, a new line of defense was proposed and considered to be specific for the control of a specialized fungal parasite of the ants’ fungus gardens (Escovopsis). However, recent studies have questioned the specificity of the integumental bacteria, as they were also found to inhibit a range of fungi, including entomopathogens. The microbiota associated with the leaf-cutting ant gardens has also been proposed as another level of chemical defense, protecting the garden from parasite invasion. Here we review the chemical defense weaponry deployed by leaf-cutting ants against parasites of their fungus gardens and of the ants themselves.
Introduction
Leaf-cutting ants are pests of major importance in Latin America due to the damage their activities cause to agricultural production, pasturelands and forestry. Despite the preference for certain species of plants, many crops are attacked by these ants, which are responsible for losses of up to 50% in forestry production.Citation1
These insects belong to a group of derived ants which are divided into two genera: Atta and Acromyrmex (Myrmicinae, Attini), with 15 and 24 species described respectively. The plant material collected by the ants is used as a substrate for the symbiotic fungus (Basidiomycete) which they cultivate in underground gardens.Citation2 This association between the ants and their fungi has now evolved to a level so complex that the components of this symbiosis are no longer able to survive separately. The fungus depends on the ants for: (1) supply of plant material essential for its growth; (2) propagation; (3) dispersion and (4) removal of invading microorganisms (weeding), whist the fungus is the sole source of nutrients for the queens, larvae and allates.Citation3 The fungus also provides nutrients to worker ants, supplementing the nutrients obtained from plant sap, to varying extents, according to different authors.Citation4-Citation6
Leaf-cutter colonies can contain millions of ants, consuming huge quantities of plant material for the cultivation of their fungal gardens. However, one of the disadvantages of living in large colonies is the constant risk of rapid spread of disease between nest-mates; therefore social insects have developed effective means to counter this menace. In order to protect the colony from parasites, the ants have developed complex defense mechanisms. These mechanisms can be divided into (1) behavioral defenses and (2) chemical defenses.
Behavioral defense mechanisms include self- and allo-grooming which are very important in the removal of pathogens from the integument.Citation7 Moreover, when ants are perceived to have contracted a disease; they are usually expelled from the colony.Citation3
Chemical defenses against parasites include the secretion of antibiotic compounds from the metapleural glands,Citation8 the production of antibiotics by bacteria associated with the integumentCitation9,Citation10 and the production of antibiotics by bacteria present in the fungus garden.Citation11 In the current review we will concentrate on the chemical weaponry deployed by the ants and their symbiotic microbiota.
The recently discovered relationship between symbiotic actinobacteria and certain species of leaf-cutting antsCitation9 has been proposed as a classic example of co-evolution.Citation12 However, the co-evolution of these organisms is now considered controversial, as some of the data that have been presented to prove this phenomenon have been questioned (for review see ref. Citation13).
The symbiotic bacteria isolated from the integument of Acromyrmex workers were initially described as belonging to the genus Streptomyces.Citation9 Subsequently, this bacterium was re-identified by sequencing and found to be of the genus Pseudonocardia.Citation14 Initially the role of Pseudonocardia was described as a mutualistic agent, acting in defense of the colonies, producing antibiotics against parasitic fungi of the genus Escovopsis (Hypocreaceae, Hypocreales), which specifically attacks the fungus cultivated by ants.Citation9 Pseudonocardia are located in the crypts associated with exocrine glands on the cuticle of the ants. The distribution of these cavities varies with gender and species. The presence of these specialized structures on the ants’ cuticle has been suggested to indicate an ancient co-evolutionary relationship of between the bacteria, ants, their symbiotic fungus and Escovopsis.Citation15
However, studies have shown that it is possible to isolate a variety of actinomycetes from the integument of leaf-cutters and that these microbes possess generalized anti-microbial activity, questioning the key assumptions of ant–Pseudonocardia–Escovopsis co-evolution.Citation16 Moreover, recent studies by Sen and coworkersCitation17 reevaluated the role of Pseudonocardia, questioning its specificity, and revealed that it possesses a wide range of action against saprophytic, phytopathogenic and entomopathogenic fungi.
Interestingly, no biological control method has yet been developed for the management of leaf-cutting ants. Entomopathogenic fungi infect insects by penetrating the integument.Citation18 However, in the case of leaf-cutting ants, these fungi would be required to overcome or disrupt the social organization and chemical defenses of the colonies.Citation19
As the control of ants by entomopathogenic agents may be hindered by the various defense mechanisms found in the colonies, primarily by the shield provided by the symbiotic integumental bacterial bio-films, the study of microorganisms associated with ants may contribute to the development of new microbial control strategies, enabling entomopathogens to become more efficient, thereby reducing ant populations to levels below the economic damage threshold.
Following this line of reasoning, the application of antibiotics may be an effective strategy against bacteria present on the exoskeleton of the ants, facilitating subsequent infection by entomopathogenic fungi and, thus increasing the potential of these agents for use in biological control of ants.
Behavioral Defenses Against Ant Pathogens
The social organization of ants is considered an effective defense strategy against invaders of their colonies, as these insects are considered to be a super-organism, where their behavioral cooperation through the division of tasks between castes complicates the spread of diseases within the colony.Citation20
Hughes et al.Citation21 studied Acromyrmex echinator and found that when the ants were exposed to conidia of Metarhizium anisopliae, the disease transmission rate was inversely related to the population density of the ants. Thus higher populations favored greater survival rates when challenged by pathogens. This would appear to be paradoxical. However when taking into consideration the defense mechanisms: self-grooming, allogrooming and the production of antibiotic compounds, there is a net benefit gained by group living as opposed to non-social insects. Thus the colony should be considered as super-organism, with greater numbers of ants living in a cooperative regime leading to higher levels of disease resistance.
Currie and StuartCitation22 observed the grooming behavior of workers of Atta colombica when exposed to pathogen contamination (Trichoderma viride and Escovopsis) of their nests. Two types of worker activities were observed: garden grooming (fungus grooming) of pathogenic spores and wholesale removal of infected parts of the garden (weeding). Furthermore, individual hygienic procedures such as self-grooming were observed. These prophylactic procedures occurred shortly after the introduction of pathogens, indicating that they were the primary defenses within an infected colony. Grooming (self- and fungus-grooming) basically involves licking the surfaces to be cleaned and the storage of contaminated particles in the infrabucal cavity. The residues in the infrabucal cavity are then transported to the waste chamber where they are regurgitated as “pellets.”Citation23 Galvanho and colleaguesCitation23 quantified self-grooming in Acromrymex subterraneus subterraneus workers exposed to conidia of the entomopathogenic fungus Beauveria bassiana. They demonstrated that workers exposed to the fungus spend significantly longer periods self-grooming than control treated workers.
The division of the nest into chambers dedicated to the deposition of unwanted materials is considered as another important prophylactic strategy adopted by ants, since the waste material is isolated from the fungus garden on which the ants feed.Citation24,Citation25 This waste material is highly contaminated with fungi, bacteria, mites and un-utilizable plant material.
Ballari et al.Citation26 noted behavioral modifications among workers which performed different tasks such as removal and disposal of garbage, management and maintenance of the symbiotic fungus and cutting and transporting fresh leaves in colonies of Acromyrmex lobicornis. Workers responsible for waste management had no access to the fungus chambers and were prevented from transiting the same trail as ants transporting cut leaves. Exposure to waste led to a 60% increase in the mortality rate of waste-heap workers compared with workers not exposed to waste.Citation27
Chemical Defense: The Role of Gland Secretions
Chemicals used to defend colonies include mandibular gland secretions, which can minimize the action of toxic compounds such as tannins and terpenoids, as well as inhibiting the germination of spores of plant pathogens such as Botrytis cinerea.Citation28,Citation29 However, these secretions were not able to inhibit the germination of fungi such as Escovopsis weberi and Metarhizium anisopliae. The bioactive components of Atta mandibular gland secretions, which are also considered to be pheromones (Citral and 4-methyl-3-Heptanol etc.), were shown to inhibit a range of microorganisms.Citation30
Secretions produced by other glands commonly found in ants have been reported to inhibit the entomopathogen M. anisopliae. The metapleural gland produced antifungal compounds in workers of Acromyrmex octospinosus.Citation31 The compounds secreted by metapleural glands can be licked and spread to the rest of the coat during self-grooming behavior, and this activity was observed in ants of the genera Atta and Acromyrmex, with a significant increase in this behavior in the presence of infectious agents such as E. weberi and M. anisopliae (garden parasites and entomopathogens).Citation32
The role of these glands is noteworthy, as there appears to be a direct relationship (or division of labor) between the use of the glands and bacterial bio-films. Studies have shown that leaf-cutting ants which do not support obvious bacterial blooms on their integument rely on the metapleural glands as a first line of defense. Fernández-Marín et al.Citation33 demonstrated that Atta and Sericomyrmex workers lacking visible bacteria bio-films responded to pathogen challenges by increasing metapleural gland grooming rates, whereas Acromyrmex and Trachymyrmex (which maintain abundant bacteria bio-films) displayed lower metapleural glands grooming rates. Poulson et al.Citation34 experimentally blocked the metapleural glands of Acromyrmex octospinosus, resulting in increased susceptibility to M. anisopliae. As the metapleural glands are larger in minor workers, this caste has been stated to be more important in Acromyrmex colony defense than major workers.Citation35 Interestingly minor workers involved in garden maintenance do not support obvious bacterial bio-films.
Poulsen et al.Citation36 investigated the effects of metapleural glands on the growth of Pseudonocardia bacteria on the integument of A. octospnosus workers. It was found that in ants with artificially blocked metapleural glands, the development of symbiotic bacteria was not affected in the exponential growth phase but during the decline phase there was a significant reduction in the bacterial biofilm, suggesting that the presence of the actinobacteria on the integument can be regulated by the metapleural gland.
Integumental Bacterial Ecto-Symbionts Protect the Ants, Their Gardens or Both?
In 1999, the white powdery substance observed on the integument of leaf-cutting ants () previously considered a cuticular exudateCitation37 was discovered to be a filamentous bacterium.Citation9 This bacterium was hailed as the third level of symbiosis within colonies of ants, producing compounds that specifically inhibited the development of the specialized garden parasite Escovopsis.Citation9
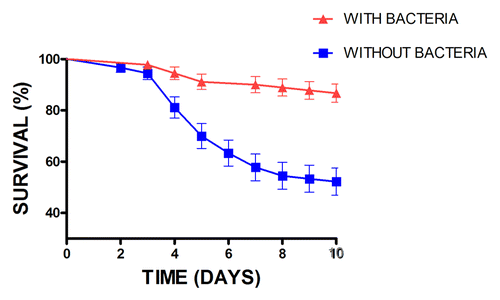
Figure 1. Survival curves of A. subterraneus subterraneus workers without bacterial biofilms (pre-treated with gentamicin) or with biofims (pre-treated with sterile distilled water) following exposure to the entomopathogenic fungus Metarhizium anisopliae. Error bars: SEM (Adapted from Mattoso et al., ref. Citation10)
Poulsen et al.Citation38 studied the biology of symbiotic bacteria on the integument of A. octospinosus major workers and observed that the bacteria first appears a few days after adult emergence, fully covering their integuments between 10 to 15 d old. Around 25 d after emergence, the workers mature and begin to forage. During this period there is a decline in the visible bacterial population on the exoskeleton.
In the case of the leaf-cutting ant Acromyrmex subterraneus subterraneus, during the first 12–15 d of the adult phase, the worker ant integument supports an easily visible and extensive bacterial coating. These ants do not, as a general rule, leave the nest to forage but are involved in garden maintenance and nursery activities. Although they do not forage, they would still be exposed to pathogens on plant material brought into the nest by foragers. These young workers represent a high level of investment to the colony and thus need to be protected from pathogens.
The pattern of biofilm development in relation to worker size, age and function may reflect the role of the bacteria. Unpublished observations by Poulsen and coworkers and by our group have shown that major workers emerge without any visible bacterial growth on their cuticle but within a few days acquire an extensive covering, which reaches a peak 10-25 d after eclosion. Subsequently, at about 25 d post-ecolsion, when major workers start to leave the colony to forage, the bacterial population declines, until it is only observed on the laterocervical plates. The fact that the actinomycete bacterium is most abundant on the major workers tending the garden has been used to support its role in garden hygiene as well as the observation of increased bacterial abundance on workers from colonies experimentally infected with Escovopsis.Citation39 Currie et al.Citation39 also treated ants with antibiotics to remove the bacterial bio-film and subsequently demonstrated an increase in the prevalence of Escovopsis infestations.
In the first empirical study of the role of the bacterial bio-film in the protection of the ants against entomopathogens, removal of the bacterial bio-film with antibiotics significantly increased the susceptibility of the ants to infection by M. anisopliae ().Citation10 It is probable that anti-fungal compounds secreted by the pseudonocardiaceous bacteria are capable to inhibiting the germination of M. anisopliae, thus preventing penetration of the integument and subsequent host colonization. Treatment of ants with UV light was inefficient in eliminating the bacterial biofilms and the ants remained protected from attack by M. anisopliae (unpublished data). We have also shown that foraging ants with reduced bacterial bio-films are more susceptible to fungal infection than younger workers of the same caste (unpublished data).
Figure 2. Pseudonocardia biofilms on the integument of Acromyrmex subterraneus subterraneus workers. (A) Young worker with high level of biofilm coverage. (B) Older worker with reduced biofilm coverage on the latero-cervical plates and lower parts of the head capsule.
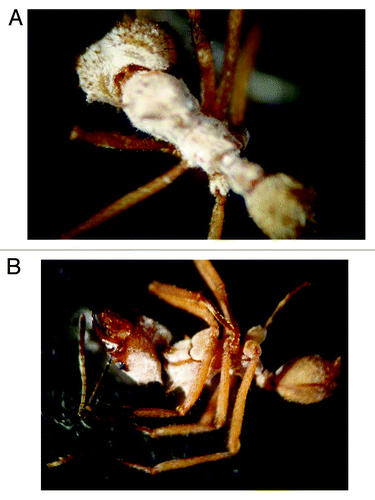
Recently two models of actinobacteria transmission between generations and between colonies of ants have been proposed.Citation40 The first actinobacteria transmission model was proposed following the discovery that the same strain of bacteria found on the integument of workers, could also be isolated from female reproductives and queens, supporting a model of vertical transmission of a single bacterial isolate between colonies. In contrast, strains of Pseudonocardia symbionts found on the integument of Acromyrmex octospinosus and Acromyrmex echinatior workers from colonies occupying the same geographical area showed no genetic variation indicating horizontal transmission of these strains.Citation41 This model establishes that actinobacteria may be obtained or changed through the generations by contact with members of other colonies or strains found in the environment.Citation41 Poulsen et al.Citation41 also noted that several strains of Pseudonocardia isolated from attine ants are phylogenetically similar to those commonly found in soil.
According to evolutionary theory, the maintenance of a single isolate of Pseudonocardia would be beneficial to the ants, since the culture of different strains could generate competition and conflict. However, the maintenance of a single isolate of Pseudonocardia, would provide little flexibility in the garden defense against infections by different isolates of Escovopsis.Citation42 Other recent evidence further reinforces the horizontal transmission model. Actinobacteria were isolated not only from reproductive females, but also from males of the fungus-growing attines, Cyphomyrmex wheeleri, supporting the idea of horizontal transmission among neighboring nests during the nuptial flight of the ants.Citation17
Sen et al.Citation17 isolated not only Pseudonocardia but also bacteria of the genus Amycolatopsis from the integument of leaf-cutting ants. The same authors also demonstrated that multiple strains may be isolated from the cuticle of only one ant. Zhang and colleaguesCitation43 offered the ants a choice between their own isolate and an actinobacteria isolated from another ant species, both of the genus Acromyrmex. It was observed that 30 to 40% of the ants chose strains of other species, indicating flexibility in acquisition of the symbiont.
Questioning Co-Evolution of Leaf-Cutters and Actinobacteria
In order to reassess the co-evolution of the ants and actinomycetes, Mueller and colleaguesCitation37 called attention to some important points such as: (1) the accumulation of microorganisms on the surface of Attini integument suggesting adaptation to an open or semi-open system, therefore allowing the acquisition of microbiota continuously from the environment, instead of a long-term association by sequestration of a specific population of microorganisms; (2) the location of actinomycete on the surface of body structures such as the thorax, legs, gaster, pronotum and pro-pleural plates indicating that the system is adapted to regularly acquire or replace the microbiota; (3) the rarity or absence of Pseudonocardia in Atta.
Recently bioactive compounds from Pseudonocardia, Streptomyces and Dermacoccus bacteria have been isolated, purified and characterized.Citation44 Haeder et al.Citation44 found that of the microorganisms associated with Acromyrmex, only bacteria of the genus Streptomyces produced a metabolite with a high inhibitory activity against Escovopsis. This was identified as a candicidin macrolide, which had no inhibitory activity against the fungal symbiont of ants. Another study demonstrated a compound obtained from Pseudonocardia isolated from Apterostigma dentigerum cuticle, called dentigerumycin capable of inhibiting the growth of Escovopsis.Citation45,Citation46
RibeiroCitation47 succeeded in isolating several strains of Streptomyces present on the cuticle of workers of Atta sexdens. These bacteria exhibited broad antimicrobial activity. Several species of yeasts were isolated from the gardens of Atta texana and subsequently tested against Escovopsis and other species of pathogenic and saprophytic fungi.Citation48 The yeast inhibited the growth of these fungi, revealing that this ant species uses a different weapon against colony invaders.
In the study by Zucchi and colleagues,Citation49 a range of bacteria were isolated from the cuticle of various attines. These bacteria inhibited Escovopsis, showing that this parasite is not specifically inhibited by Pseudonocardia. Sen and colleaguesCitation17 evaluated the inhibitory activity of a variety of bacteria of the genus Pseudonocardia against other microorganisms other than Escovopsis, such as the entomopathogenic fungi B. bassiana, M. anisopliae and Acrodontium sp saprophytic fungi Cyphellophora sp and Alternaria tenuissima, as well as the symbiotic fungus cultivated by ants (Leucoagaricus). Different levels of inhibition against all of these microorganisms were observed. The same authors found that not only Pseudonocardia, but other bacteria such as Amycolatopsis may occupy similar regions in the integument of attine ants.
Although the so called “pseudonocardiaceous” associates of attine workers may be involved primarily in protecting the fungus garden against Escovopsis invasion, as suggested by a wealth of published data,Citation13 these bacteria could also have a role in the protection of the colony as a whole.
This possibility was in fact first raised by Currie et al.Citation39 when trying to explain the observation that minor workers (specialized in tending the garden) had a lower abundance of actinomycetes on their cuticles as compared with major workers. Due to the previous finding that the actinomycete does not produce secondary metabolites with general anti-fungal properties, which would help protect workers from ubiquitous entomopathogenic fungi,Citation9 they suggested that the bacterial bio-film could serve as a physical barrier preventing fungal spores from coming in contact with the exoskeleton of the ants.
The fact that the actinomycete bacterium is most abundant on the major workers tending the garden has been used to support its role in garden hygiene as well as the observation of increased bacterial abundance on workers from colonies experimentally infected with Escovopsis.Citation39 Currie et al.Citation39 treated ants with antibiotics and subsequently showed an increase in the prevalence of Escovopsis in mini-colonies. Our group demonstrated that removal of the bacterial biofilm with antibiotics significantly increases the susceptibility of the ants to infection by M. anisopliae ().Citation10 It is probable that anti-fungal compounds secreted by the pseudonocardiaceous bacteria are capable of inhibiting the germination of M. anisopliae, thus preventing penetration of the integument and host colonization. Not only does the removal of the biofilm facilitate cuticle penetration by entomopathogenic fungi but also increased success of the fungus during the later stages of colonization, as seen from the results for conidio-genesis (unpublished results). Interestingly the removal of the bio-film in non-fungus treated ants facilitated the colonization of cadavers by saprophytic fungi further supporting the role of the bacteria in inhibiting contaminating microbes. We have also shown that foraging ants with naturally low levels of bacterial bio-film coverage are highly susceptible to fungal infection (unpublished results).
Extensive symbiotic bacterial bio-films present over the first weeks of adult life could protect these vulnerable colony members. Younger workers serve the needs of the colony for a longer time period and thus represent a high investment. To protect this investment, the younger workers and female reproductive alates typically have the highest abundance of actinomycete on their integument.Citation39
The results presented here support a role of pseudonocardiaceous bacteria as a first line of defense against fungal pathogen attack. Normally the first line of defense of any arthropod is the integument, an effective barrier for pesticides, predators and pathogens. In the case of Acromyrmex, this defensive barrier is reinforced with anti-fungal secreting bacteria.
What is the Role of the Garden Microbiota?
In the not too distant past, leaf-cutting ant gardens were considered to be a mono-culture of the symbiotic fungus Leucoagaricus gongylophorus (Family Lepiotaceae), which the ants maintained meticulously clean and free of any contaminating microorganisms.Citation3 However, this hypothesis has now been radically reconsidered as an ever increasing diversity of microorganisms is regularly being isolated from the fungus gardens of leaf-cutting ants and there is every reason to believe that they are not simple contaminants.Citation13
Santos and coworkersCitation11 demonstrated the occurrence of the bacterium Burkholderia sp in Atta sexdens colonies. PCR of 16S and 23S rDNA genes clearly identified the ant-associated isolates as B. cepacia. However, because taxonomy of the B. cepacia complex is complicated, Santos et al.Citation11 preferred to describe the ant-associated bacteria simply as Burkholderia sp B. cepacia is a Gram-negative soil bacterium commonly associated with plants, although certain members of the complex are also opportunistic human pathogens.Citation50,Citation51 Bacteria of the B. cepacia complex are well known to produce antifungal compoundsCitation52 and have been proposed as biocontrol agents for soil-borne plant pathogens.Citation53 The presence of Burkholderia sp in young nests of A. sexdens rubropilosa, may play an important role in their defense against pathogenic microorganisms.
The use of a range of antimicrobial compounds is considered as a strategy to avoid evolution of resistant pathogens, in an analogy with human health care, in which using mixing rather than cycling of antibiotics has been predicted to avoid resistance development.Citation54
Burkholderia sp was frequently isolated from the nests of leaf cutting ants. The prevalence of this bacterium within the fungal garden of young colonies suggests that the consortium of microorganisms involved in the ant-fungal garden is even more complex than previously envisaged. This bacterium inhibited development of Escovopsis, the entomopathogenic fungi M. anisopliae and B. bassiana and the saprophyte Verticillium lecanii. Yeasts isolated from garden bio-films also inhibited Escovopsis and may serve in protection against disease in attine gardens.Citation55
Attine ants may have developed multiple biological sources of antibiotics, with activity against microbial agents of diseases of the ants themselves and competitors of their mutualist fungus 45–65 million years before the origins of human agriculture or medicine.Citation56
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Oliveira MA, Araújo MS, Marinho CGS, Ribeiro MMRR, Della Lucia TMC. Manejo de formigas-cortadeiras. In: (Della Lucia TMC ed.) Formigas cortadeiras: da bioecologia ao manejo. 1. ed. Viçosa: Editora UFV 2011; 420p.
- Mueller UG, Gerardo NM, Aanen DK, Six DL, Schultz TR. The evolution of agriculture in insects. Annu Rev Ecol Evol Syst 2005; 36:563 - 95; http://dx.doi.org/10.1146/annurev.ecolsys.36.102003.152626
- Hölldobler B, Wilson EO. The ants. 1990; 1st ed. Cambridge: Harvard University Press.
- Littledyke M, Cherrett JM. Direct ingestion of plant sap from cut leaves by the leaf-cutting ants Atta cephalotes and Acromyrmex octospinosus (Hymenoptera: Formicidae). Bull Entomol Res 1978; 68:263 - 71; http://dx.doi.org/10.1017/S0007485300007343
- Quinlan RJ, Cherrett JM. The role of the fungus in the diet of the leaf-cutting ant Atta cephalotes.. Ecol Entomol 1979; 4:151 - 60; http://dx.doi.org/10.1111/j.1365-2311.1979.tb00570.x
- Silva A, Bacci M Jr., Gomes de Siqueira C, Correa Bueno O, Pagnocca FC, Aparecida Hebling MJ. Survival of Atta sexdens workers on different food sources. J Insect Physiol 2003; 49:307 - 13; http://dx.doi.org/10.1016/S0022-1910(03)00004-0; PMID: 12769984
- Morelos-Juárez C, Walker TN, Lopes JFS, Hughes WOH. Ant farmers practice proactive personal hygiene to protect their fungus crop. Curr Biol 2010; 20:R553 - 4; http://dx.doi.org/10.1016/j.cub.2010.04.047; PMID: 20619804
- Bot ANM, Ortius-Lechner D, Finster K, Maile R, Boomsma JJ. Variable sensitivity of fungi andbacteria to compounds produced by the metapleural glands of leaf-cutting ants. Insectes Soc 2002; 49:363 - 70; http://dx.doi.org/10.1007/PL00012660
- Currie CR, Scott JA, Summerbell RC, Malloch D. Fungus-growing ants use antibiotic-producing bacteria to control garden parasites. Nature 1999; 398:701 - 4; http://dx.doi.org/10.1038/19519
- Mattoso TC, Moreira DDO, Samuels RI. Symbiotic bacteria on the cuticle of the leaf-cutting ant Acromyrmex subterraneus subterraneus protect workers from attack by entomopathogenic fungi. Biol Lett 2012; 8:461 - 4; http://dx.doi.org/10.1098/rsbl.2011.0963; PMID: 22130174
- Santos AV, Dillon RJ, Dillon VM, Reynolds SE, Samuels RI. Ocurrence of the antibiotic producing bacterium Burkholderia sp. in colonies of the leaf-cutting ant Atta sexdens rubropilosa. FEMS Microbiol Lett 2004; 239:319 - 23; http://dx.doi.org/10.1016/j.femsle.2004.09.005; PMID: 15476982
- Currie CR. A community of ants, fungi, and bacteria: a multilateral approach to studying symbiosis. Annu Rev Microbiol 2001; 55:357 - 80; http://dx.doi.org/10.1146/annurev.micro.55.1.357; PMID: 11544360
- Mueller UG. Symbiont recruitment versus ant-symbiont co-evolution in the attine ant-microbe symbiosis. Curr Opin Microbiol 2012; 15:269 - 77; http://dx.doi.org/10.1016/j.mib.2012.03.001; PMID: 22445196
- Cafaro MJ, Currie CR. Phylogenetic analysis of mutualistic filamentous bacteria associated with fungus-growing ants. Can J Microbiol 2005; 51:441 - 6; http://dx.doi.org/10.1139/w05-023; PMID: 16121221
- Currie CR, Poulsen M, Mendenhall J, Boomsma JJ, Billen J. Coevolved crypts and exocrine glands support mutualistic bacteria in fungus-growing ants. Science 2006; 311:81 - 3; http://dx.doi.org/10.1126/science.1119744; PMID: 16400148
- Kost C, Lakatos T, Böttcher I, Arendholz WR, Redenbach M, Wirth R. Non-specific association between filamentous bacteria and fungus-growing ants. Naturwissenschaften 2007; 94:821 - 8; http://dx.doi.org/10.1007/s00114-007-0262-y; PMID: 17541536
- Sen R, Ishak HD, Estrada D, Dowd SE, Hong E, Mueller UG. Generalized antifungal activity and 454-screening of Pseudonocardia and Amycolatopsis bacteria in nests of fungus-growing ants. Proc Natl Acad Sci U S A 2009; 106:17805 - 10; http://dx.doi.org/10.1073/pnas.0904827106; PMID: 19805175
- Charnley AK. Entomopathogenic fungi and their role in pest control. In: Wicklow D.T., Soderstrom M., (eds) The mycota IV - environmental and microbial relationships. Berlim Heidelberg: Springer-Verlag 1997; p. 185-201.
- Santos AV, de Oliveira BL, Samuels RI. Selection of entomopathogenic fungi for use in combination with sub-lethal doses of imidacloprid: perspectives for the control of the leaf-cutting ant Atta sexdens rubropilosa Forel (Hymenoptera: Formicidae). Mycopathologia 2007; 163:233 - 40; http://dx.doi.org/10.1007/s11046-007-9009-8; PMID: 17404893
- Wilson EO. The Insect Societies. 1. ed. Cambridge: Harvard University 1971; 548p.
- Hughes WOH, Eilenberg J, Boomsma JJ. Trade-offs in group living: transmission and disease resistance in leafcutting ants. Proc R Soc Lond B 2002; 269: 1811-19.
- Currie CR, Stuart AE. Weeding and grooming of pathogens in agriculture by ants. Proc Biol Sci 2001; 268:1033 - 9; http://dx.doi.org/10.1098/rspb.2001.1605; PMID: 11375087
- Little AEF, Murakami T, Mueller UG, Currie CR. Defending against parasites: fungus-growing ants combine specialized behaviours and microbial symbionts to protect their fungus gardens. Biol Lett 2006; 2:12 - 6; http://dx.doi.org/10.1098/rsbl.2005.0371; PMID: 17148313
- Galvanho JP, Carrera MP, Moreira DDO, Erthal M Jr., Silva CP, Samuels RI. Imidacloprid Inhibits Behavioral Defenses of the Leaf-Cutting Ant Acromyrmex subterraneus subterraneus (Hymenoptera:Formicidae). J Insect Behav 2012; In press http://dx.doi.org/10.1007/s10905-012-9328-6
- Currie CR, Stuart AE. Weeding and grooming of pathogens in agriculture by ants. Proc Biol Sci 2001; 268:1033 - 9; http://dx.doi.org/10.1098/rspb.2001.1605; PMID: 11375087
- Hart AG, Ratnieks FLW. Waste management in the leaf-cutting ant Atta colombica.. Behav Ecol 2002; 13:224 - 31; http://dx.doi.org/10.1093/beheco/13.2.224
- Ballari S, Farji-Brener A, Tadey M. Waste management in the leaf-cutting ant Acromyrmex lobicornis: division of labour, aggressive behaviour, and location of external refuse dumps. J Insect Behav 2007; 20:87 - 98; http://dx.doi.org/10.1007/s10905-006-9065-9
- Brown MJ, Bot AN, Hart AG. Mortality rates and division of labor in the leaf-cutting ant, Atta colombica.. J Insect Sci 2006; 6:1 - 8; http://dx.doi.org/10.1673/2006_06_18.1; PMID: 19537995
- Knapp JJ, Jackson CW, Howse PE, Vilela EF. Mandibular gland secretions of leaf-cutting ants: role in defense against alien fungi. XII Congress of the International Union for the Study of Social Insects 1994; Paris: p. 109.
- Marsaro AL Jr., Della Lucia TMC, Barbosa LCA, Maffia LA, Morandi MAB. Efeito de secreções da glândula mandibular de Atta sexdens rubropilosa Forel (Hymenoptera: Formicidae) sobre a germinação de conídios de Botrytis cinerea Pers. Fr. Neotrop Entomol 2001; 30:403 - 6; http://dx.doi.org/10.1590/S1519-566X2001000300010
- Mendonça AdeL, da Silva CE, de Mesquita FL, Campos RdaS, Do Nascimento RR, Ximenes ECPA, et al. Antimicrobial activities of components of the glandular secretions of leaf cutting ants of the genus Atta.. Antonie Van Leeuwenhoek 2009; 95:295 - 303; http://dx.doi.org/10.1007/s10482-009-9312-0; PMID: 19214771
- Bot ANM, Ortius-Lechner D, Finster K, Maile R, Boomsma JJ. Variable sensitivity of fungi and bacteria to compounds produced by the metapleural glands of leaf-cutting ants. Insectes Soc 2002; 49:363 - 70; http://dx.doi.org/10.1007/PL00012660
- Fernández-Marín H, Zimmerman JK, Rehner SA, Wcislo WT. Active use of the metapleural glands by ants in controlling fungal infection. Proc Biol Sci 2006; 273:1689 - 95; http://dx.doi.org/10.1098/rspb.2006.3492; PMID: 16769642
- Fernández-Marín H, Zimmerman JK, Nash DR, Boomsma JJ, Wcislo WT. Reduced biological control and enhanced chemical pest management in the evolution of fungus farming in ants. Proc Biol Sci 2009; 276:2263 - 9; http://dx.doi.org/10.1098/rspb.2009.0184; PMID: 19324734
- Poulsen M, Bot ANM, Nielsen MG, Boomsma JJ. Experimental evidence for the costs and hygienic significance of the antibiotic metapleural gland secretion in leaf-cutting ants. Behav Ecol Sociobiol 2002; 52:151 - 7; http://dx.doi.org/10.1007/s00265-002-0489-8
- Poulsen M, Bot ANM, Currie CR, Boomsma JJ. Mutualistic bacteria and a possible trade-off between alternative defense mechanisms in Acromyrmex leaf-cutting ants. Insectes Soc 2002; 49:15 - 9; http://dx.doi.org/10.1007/s00040-002-8271-5
- Poulsen M, Bot ANM, Boomsma JJ. The effect of metapleural gland secretion on the growth of a mutualistic bacterium on the cuticle of leaf-cutting ants. Naturwissenschaften 2003; 90:406 - 9; http://dx.doi.org/10.1007/s00114-003-0450-3; PMID: 14504783
- Mueller UG, Dash D, Rabeling C, Rodrigues A. Coevolution between attine ants and actinomycete bacteria: a reevaluation. Evolution 2008; 62:2894 - 912; http://dx.doi.org/10.1111/j.1558-5646.2008.00501.x; PMID: 18752608
- Poulsen M, Bot ANM, Currie CR, Nielsen MG, Boomsma JJ. Within-colony transmission and the cost of a mutualistic bacterium in the leaf-cutting ant Acromyrmex octospinosus.. Funct Ecol 2003; 17:260 - 9; http://dx.doi.org/10.1046/j.1365-2435.2003.00726.x
- Currie CR, Bot ANM, Boomsma JJ. Experimental evidence of a tripartite mutualism: bacteria protect ant fungus gardens from specialized parasites. Oikos 2003; 101:91 - 102; http://dx.doi.org/10.1034/j.1600-0706.2003.12036.x
- Boomsma JJ, Aanen DK. Rethinking crop-disease management in fungus-growing ants. Proc Natl Acad Sci U S A 2009; 106:17611 - 2; http://dx.doi.org/10.1073/pnas.0910004106; PMID: 19826091
- Poulsen M, Cafaro M, Boomsma JJ, Currie CR. Specificity of the mutualistic association between actinomycete bacteria and two sympatric species of Acromyrmex leaf-cutting ants. Mol Ecol 2005; 14:3597 - 604; http://dx.doi.org/10.1111/j.1365-294X.2005.02695.x; PMID: 16156826
- Poulsen M, Erhardt DP, Molinaro DJ, Lin TL, Currie CR. Antagonistic bacterial interactions help shape host-symbiont dynamics within the fungus-growing ant-microbe mutualism. PLoS One 2007; 2:e960; http://dx.doi.org/10.1371/journal.pone.0000960; PMID: 17896000
- Zhang MM, Poulsen M, Currie CR. Symbiont recognition of mutualistic bacteria by Acromyrmex leaf-cutting ants. ISME J 2007; 1:313 - 20; PMID: 18043642
- Haeder S, Wirth R, Herz H, Spiteller D. Candicidin-producing Streptomyces support leaf-cutting ants to protect their fungus garden against the pathogenic fungus Escovopsis.. Proc Natl Acad Sci U S A 2009; 106:4742 - 6; http://dx.doi.org/10.1073/pnas.0812082106; PMID: 19270078
- Oh D-C, Scott JJ, Poulsen MZ, Currie CR, Clardy J. Discovery of new secondary metabolites mediating insect-microorganism symbioses. Planta Med 2008; 74:906 - 06; http://dx.doi.org/10.1055/s-0028-1083882
- Oh D-C, Poulsen M, Currie CR, Clardy J. Dentigerumycin: a bacterial mediator of an ant-fungus symbiosis. Nat Chem Biol 2009; 5:391 - 3; http://dx.doi.org/10.1038/nchembio.159; PMID: 19330011
- Ribeiro SB. Caracterização de espécies bacterianas encontradas em ninhos de Atta sexdens L. e isolamento de Streptomyces de formigas da tribo Attini. PhD Thesis Rio Claro SP, 2000 Universidade Estadual Paulista.
- Rodrigues AR, Cable RN, Mueller UG, Bacci M Jr., Pagnocca FC. Antagonistic interactions between garden yeasts and microfungal garden pathogens of leaf-cutting ants. Antonie Van Leeuwenhoek 2009; 96:331 - 42; http://dx.doi.org/10.1007/s10482-009-9350-7; PMID: 19449210
- Zucchi TD, Guidolin AS, Cônsoli FL. Isolation and characterization of actinobacteria ectosymbionts from Acromyrmex subterraneus brunneus (Hymenoptera, Formicidae). Microbiol Res 2011; 166:68 - 76; http://dx.doi.org/10.1016/j.micres.2010.01.009; PMID: 20171857
- Parke JL, Gurian-Sherman D. Diversity of the Burkholderia cepacia complex and implications for risk assessment of biological control strains. Annu Rev Phytopathol 2001; 39:225 - 58; http://dx.doi.org/10.1146/annurev.phyto.39.1.225; PMID: 11701865
- Coenye T, Vandamme P. Diversity and significance of Burkholderia species occupying diverse ecological niches. Environ Microbiol 2003; 5:719 - 29; http://dx.doi.org/10.1046/j.1462-2920.2003.00471.x; PMID: 12919407
- Lim YH, Suh JW, Kim S, Hyun BC, Kim CS, Lee CH. Cepacidine A, a novel antifungal antibiotic produced by Pseudomonas cepacia. II. Physico-chemical properties and structure elucidation. J Antibiot (Tokyo) 1994; 47:1406 - 16; http://dx.doi.org/10.7164/antibiotics.47.1406; PMID: 7531194
- Parke JL, Rand RE, Joy AE, King EB. Biological control of Pythium damping-off and Aphanomyces root rot of peas by application of Pseudomonas cepacia or P. fluorescens to seed. Plant Dis 1991; 75:987 - 92; http://dx.doi.org/10.1094/PD-75-0987
- Bergstrom CT, Lo M, Lipsitch M. Ecological theory suggests that antimicrobial cycling will not reduce antimicrobial resistance in hospitals. Proc Natl Acad Sci U S A 2004; 101:13285 - 90; http://dx.doi.org/10.1073/pnas.0402298101; PMID: 15308772
- Rodríguez A, Bacci M Jr., Mueller UG, Ortiz A, Pagnocca FC. Microfungal “Weeds” in the Leafcutter Ant Symbiosis. Microb Ecol 2008; 1:1 - 5
- Currie CR. A community of ants, fungi, and bacteria: a multilateral approach to studying symbiosis. Annu Rev Microbiol 2001; 55:357 - 80; http://dx.doi.org/10.1146/annurev.micro.55.1.357; PMID: 11544360