Abstract
The impact of symbionts on their insect hosts depends on their infection density. In the current study, we investigated the effects of host plants (cucumber, cabbage, and cotton) on the relative amount of symbionts Portiera and Hamiltonella in the whitefly Bemisia tabaci (Gennadius) Q. The relative amounts of symbionts in 3 host plant B. tabaci Q populations with the same genetic background were evaluated by quantitative PCR. The whiteflies of cabbage population harbored more Portiera than those of cucumber and cotton populations, and the relative amount of Portiera did not differ statistically between cotton and cucumber populations. The whiteflies of cucumber and cabbage populations harbored more Hamiltonella than that of cotton population, and the relative amount of Hamiltonella did not differ statistically between cabbage and cucumber populations, indicated that the relative amount of symbionts was significantly affected by host plant. In addition, the method of analyzing the composition of free amino acid in B. tabaci was established. Twenty-eight amino acids were detected in the B. tabaci Q population, the non-essential amino acids, such as glutamate, glutamine, alanine, proline and the essential amino acid arginine were the dominant amino acids in B. tabaci Q.
Keywords: :
Introduction
The sweet potato whitefly Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) is a destructive pest of many field and protected crops worldwide. B. tabaci has been regarded as a species complex consisting of many biotypes that are morphologically indistinguishable but differ in host range, feeding behavior, virus transmission, insecticide resistance, or the symbionts that they harbor.Citation1-Citation5 The two most invasive and destructive biotypes are biotype B (also known as Middle East-Minor Asia 1 genetic group, hereafter referred to as B) and biotype Q (also known as the Mediterranean genetic group, hereafter referred to as Q). During the past two decades, B. tabaci B and Q have spread from their native ranges to as many as 60 countries and resulted in serious economic losses to agricultural production worldwide.Citation6
Bemisia tabaci was first recorded in China in the late 1940s,Citation7 but the crop damages and loses caused by this insect had not been serious until the introduction of B. tabaci B in the 1990s.Citation8 Since then, B rapidly invaded the entire country, and has led to serious yield losses in many crops.Citation9,Citation10 Q was first found in Yunnan Province in 2003 and was considered a new, invasive whitefly in China.Citation10 During the past several years, Q has gradually displaced the well-established populations of B and has become the dominant form of B. tabaci in field agricultural systems in most parts of China.Citation5,Citation11
Like many other insects, B. tabaci hosts bacterial endosymbionts.Citation12 The symbionts of insects are generally divided into two groups: primary symbionts (referred to as P-symbionts) and secondary symbionts (referred to as S-symbionts).Citation12,Citation13 To date, seven symbionts (Portiera, Hamiltonella, Cardinium, Wolbachia, Fritschea, Arsenophonus, and Rickettsia) have been reported from B. tabaci.Citation14-Citation18 The P-symbiont Portiera provides nutrients that supplement the insufficient nutrients that B. tabaci obtains from its restricted diet of plant phloem,Citation12,Citation15 while S-symbionts play important roles in B. tabaci biology and ecology.Citation19-Citation24 For example, high bacterial densities in B. tabaci are correlated with the insect's ability to detoxify insecticides and other toxic compounds.Citation22
The amount of the symbiont (the number of symbionts per host whitefly) is important because it influences both the efficiency of transmission of the symbiont to the offspring and the virulence of the symbiont.Citation25 Latest research from our laboratory has recently shown that the relative amount of symbionts in insect hosts B. tabaci B changes with host-plant adaptation and insecticide resistance.Citation26
The nutritional physiology of whiteflies is governed by two linked factors; the phloem sap on which whiteflies feed, and the presence of P-symbiont. Phloem sap contains low concentrations of nitrogen in the form of free amino acids that are dominated by non-essential amino acids. The main nitrogen sources of whiteflies are free amino acids found in very low and unbalanced concentrations in the phloem sap and it is well known that whiteflies, like other animals, cannot synthesize all the amino acids.Citation12 Portiera supplement this diet through the provision of essential amino acids to the whitefly.Citation12 However, so far, only one study has studied the amino acids composition in B. tabaci using the high performance liquid chromatography (HPLC).Citation27
In the current study we: (1) measured the effect of the host plant on the relative amount of P- and S-symbionts in B. tabaci Q populations; and (2) established the method of analyzing the composition of free amino acid in B. tabaci.
Materials and Methods
Plant cultures
Three crop species from 3 families, which have been widely cultivated in China, were used in the experiments: cabbage (Brassica oleracea L., cv Jingfeng 1), cucumber (Cucumis sativus L., cv Zhongnong 12), and cotton (Gossypium herbaceum L., cv DP99B). All the 3 field crops were grown in a potting mix (a mixture of peat moss, vermiculite, organic fertilizer, perlite in a 10:10:10:1 ratio by volume) in 1.5 L pots (1 plant/pot) and enclosed in whitefly-proof screen cages under natural light and controlled temperature (26 ± 2°C) in a glasshouse. When these plants grew to the 5–7 true leaf stage, they were used to maintain the corresponding B. tabaci laboratory populations (see next section).
Bemisia tabaci Q host populations
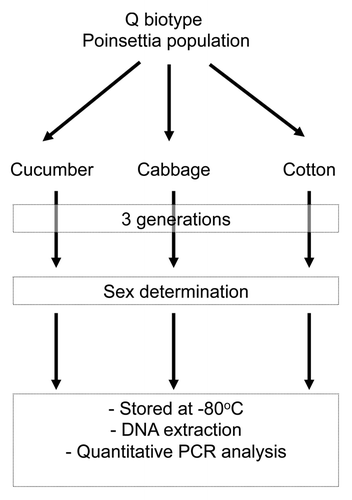
The three host populations of B. tabaci Q (cotton, cucumber, and cabbage) used in the present study were derived from the same parental population (). The parental Q whitefly population used has been maintained on poinsettia (Euphorbia pulcherrima Wild. ex. Klotz.) in isolated whitefly-proof screen cages under natural lighting and controlled temperature (26 ± 2°C) in a glasshouse.Citation5 Only the P-symbiont Portiera and S-symbiont Hamiltonella were detected from the parental population using the method of PCR and fluorescence in situ hybridizations (FISH) (Qi Su, unpublished data). The purity of biotype has been monitored and determined by the cleavage amplified polymorphic sequence (CAPS) and mitochondrial cytochrome oxidase I genes (mtCOI) for 15 adults per generation.Citation28 After three successive generations, the three host populations were used.
Quantitative real-time PCR (qPCR)
Females from each of the three populations (cabbage, cucumber and cotton) of B. tabaci Q were collected (giving 3 samples for each population with 20 females per sample), stored at -80°C, and then subjected to DNA extraction with a TIANamp Genomic DNA Kit (Tiangen Biotech Co., Ltd). The purified DNA from each sample was eluted with 200 μL of AE buffer supplied in the kit. The symbionts in the samples were quantified by qPCR according to the method of Pan et al. (2013).Citation26 The gene names, amplicon sizes and primers are listed in .
Table 1. Oligonucleotide primers used in quantitative PCR
Free amino acid analysis
Newly emerged (0–8 h post-emergence) Q whitefly adults were collected from poinsettia. Twenty mg whiteflies as one replicate that kept in 1.5 ml tube were fully homogenate, shaked for 2 min on the vortex shaker (QL-866, Qilinbeier). The sample was then centrifuged at 14,000 rpm for 10 min, 1ml supernatant was got, and was mixed with an equal volume of n-hexane, and then centrifuged at 10000 rpm for 10 min (wipe off fat), the supernatant was discarded, 0.5 ml of underlayer was got, and was mixed with an equal volume of 8% sulfosalicylic acid, and then centrifuged at 10,000 rpm for 10 min (wipe off protein), 0.5 ml of the supernatant concentrated to dryness, and 0.75 ml sample buffer was used to redissolved, and then filtrated through 0.45 μm membrane. Free amino acid in whiteflies was analyzed by amino acids analyzer (S433, sykam)
Data analysis
The free amino acids were determined by automatic amino acid analyzer. A total of 28 different peaks were detected, the peak area represents the respective amino acid content. The percentage of the various amino acids was calculated by the formula: the respective amino acid content / the total amino acids content × 100%.The differences in relative amount of symbionts in B. tabaci Q reared on three host plants were analyzed using one-way analysis of variance (ANOVA), and the means were compared by the Tukey test at p < 0.05. All statistical analyses were performed with SPSS version 17.0 (SPSS Inc.).
Results
Effects of host plant on symbionts
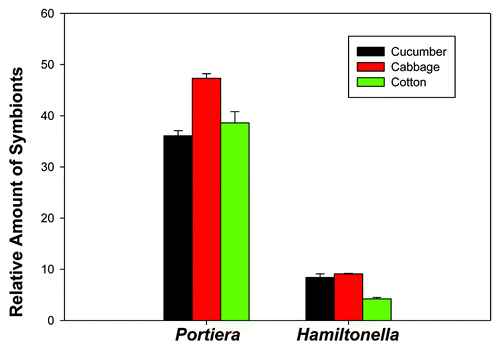
The whiteflies of cabbage population harbored more Portiera than those of cucumber and cotton populations (F2, 6 = 15.893, p = 0.004), and the relative amount of Portiera did not differ statistically between cotton and cucumber populations (). The whiteflies of cucumber and cabbage populations harbored more Hamiltonella than that of cotton population (F2, 6 = 31.765, p = 0.001), and the relative amount of Hamiltonella did not differ statistically between cabbage and cucumber populations ().
Figure 2. Relative amount of Portiera and Hamiltonella in three B. tabaci Q populations (cucumber, cabbage, and cotton) as determined by quantitative PCR (normalized according to the amount of actin gene). Values for relative amount of symbionts are means ± SEM of three replicates for each kind of plant. The data were analyzed with one-way analysis of variance. For each kind of symbiont, different letters above the bars indicate significant differences among the three populations (Tukey test, P < 0.05).
The composition and percentage of free amino acid in the B. tabaci Q population
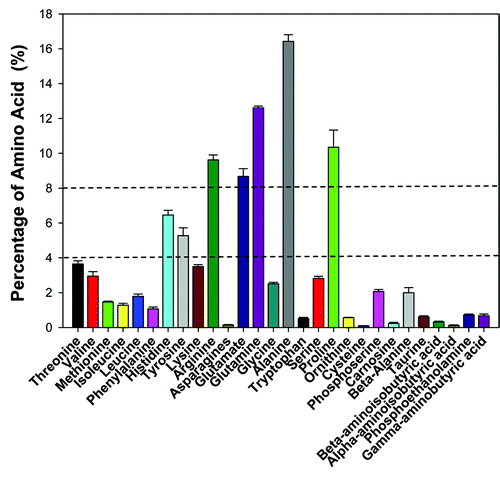
Twenty eight amino acids were detected in the B. tabaci Q population (). The non-essential amino acids, such as glutamate, glutamine, alanine, proline and the essential amino acid arginine were the dominant amino acids. The percentage of these five amino acids was all above 8.0% (). In addition, the percentage of the essential amino acids histidine and tyrosine was between 4.0–8.0% (). The percentage of the rest amino acids was all below 4.0%.
Figure 3. Free amino acids composition in the B. tabaci Q poinsettia population. Free amino acid in whiteflies was analyzed by amino acids analyzer (S433, sykam, Germany). A total of 28 different peaks were detected, the peak area represents the respective amino acid content. The percentage of the various amino acids was calculated by the formula: the respective amino acid content / the total amino acids content × 100%.
Discussion
In the current study, our survey data of the 3 host plant sub-populations derived from the same parental population of B. tabaci Q indicate that host plants substantially affects the amount of the S-symbionts in B. tabaci. This is because the amounts of P- and S-symbionts were all significantly different among the 3 host plant sub-populations. This result almost agrees with that of Pan et al. (2013)Citation26 which reports that significant variations were exhibited in the amounts of P- and S-symbionts among different host plant-adapted B. tabaci B sub-populations with the same genetic background.Citation26 Host plants exhibited marked impacts on the amount of symbionts in Q whiteflies (). However, as discussed in our latest study,Citation26 possible founder effects might result in the S-symbiont-host plant association pattern from this experiment. Additionally, other factors such as the genetic background, population inertia and stochasticity also might confound the impacts of host plants on the amount of S-symbionts and cause the discrepancies.
Previous studies showed that multiple infections, i.e., infections by different symbionts within the same individuals, are common among diverse insect taxa.Citation5,Citation29-Citation34 Although all 3 host-plant populations contained higher densities of P-symbiont Portiera than of S-symbionts Hamiltonella, the amount of each symbiont often differed among the three host populations. In particular, the amount of the Portiera was significantly affected by co-infection with the Hamiltonella in the cucumber population of B. tabaci Q. This result is consistent with that of Pan et al. (2013)Citation26 who report the amount of Portiera and S-symbionts influence each other in B. tabaci B cabbage and cucumber populations, respectively.Citation26 However, the amount of Portiera was not influenced by co-infection with S-symbionts in both B. tabaci BCitation26 and Q cabbage populations (). This disparity may be because of the fact that different plant species harbor different phytotoxin or secondary metabolites that have antibiotic effects on herbivores.
More generally, this study illustrates the useful method to analyze the amino acid composition in the small insect whitefly. Wang et al. (2012)Citation27 used the method of HPLC to analyze the free amino acids in B. tabaci, 20 free amino acids was detected in B. tabaci, however, 28 free amino acids was detected in this study. It looks like our method was easier to operate and much cheaper (), this method should be taken to evaluate the free amino acids in small insect in future. Latest study showed that Portiera was able to synthesize the essential amino acids threonine and tryptophan and the nonessential serine and lack the last gene of phenylalanine, valine, leucine, and isoleucine pathways.Citation35 Further research is needed to determine the composition of free amino acid in the three B. tabaci Q (cotton, cucumber, and cabbage) populations. If so, we can know better the relationship between the composition of acids, the amount of symbionts in B. tabaci, and the host plant.
Acknowledgments
This research was supported by the National Science Fund for Distinguished Young Scholars (31025020), the 973 Program (2013CB127602), and the Beijing Key Laboratory for Pest Control and Sustainable Cultivation of Vegetables. The granting agencies have no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Author Contributions
Y.J.Z., H.P.P., W.X., S.L.W., Q.J.W. and BYX designed the experiments. H.P.P., Q.S., L.Z. and B.M.L. performed the experiments. H.P.P., L.Z. and X.G.J. analyzed the data. H.P.P., X.G.J., Q.S. and Y.J.Z. wrote the paper.
References
- Brown JK, Frohlich DR, Rosell RC. The sweetpotato or silverleaf whiteflies: biotypes of Bemisia tabaci or a species complex?. Annu Rev Entomol 1995; 40:511 - 34; http://dx.doi.org/10.1146/annurev.en.40.010195.002455
- Perring TM. The Bemisia tabaci species complex. Crop Prot 2001; 20:725 - 37; http://dx.doi.org/10.1016/S0261-2194(01)00109-0
- Liu BM, Yan FM, Chu D, Pan HP, Jiao XG, Xie W, et al. Difference in feeding behaviors of two invasive whiteflies on host plants with different suitability: implication for competitive displacement. Int J Biol Sci 2012; 8:697 - 706; http://dx.doi.org/10.7150/ijbs.4108; PMID: 22701340
- Pan HP, Li XC, Ge DQ, Wang SL, Wu QJ, Xie W, et al. Factors affecting population dynamics of maternally transmitted endosymbionts in Bemisia tabaci.. PLoS One 2012; 7:e30760; http://dx.doi.org/10.1371/journal.pone.0030760; PMID: 22383972
- Pan HP, Chu D, Yan WQ, Su Q, Liu BM, Wang SL, et al. Rapid spread of tomato yellow leaf curl virus in China is aided differentially by two invasive whiteflies. PLoS One 2012; 7:e34817; http://dx.doi.org/10.1371/journal.pone.0034817; PMID: 22514670
- De Barro PJ, Liu SS, Boykin LM, Dinsdale AB. Bemisia tabaci: a statement of species status. Annu Rev Entomol 2011; 56:1 - 19; http://dx.doi.org/10.1146/annurev-ento-112408-085504; PMID: 20690829
- Zhou Y. The list of whiteflies in China. China Entomol 1949; 3:1 - 18
- Luo C, Yao Y, Wang RJ, Yan FM, Hu DX, Zhang ZL. The use of mitochondrial cytochrome oxidase mtCOI gene sequences for the identification of biotypes of Bemisia tabaci (Gennadius) in China. Acta Entomol Sin 2002; 45:759 - 63
- Chu D, Zhang YJ, Brown JK, Cong B, Xu BY, Wu QJ, et al. The introduction of the exotic Q biotype of Bemisia tabaci (Gennadius) from the Mediterranean region into China on ornamental crops. Fla Entomol 2006; 89:168 - 74; http://dx.doi.org/10.1653/0015-4040(2006)89[168:TIOTEQ]2.0.CO;2
- Liu SS, De Barro PJ, Xu J, Luan JB, Zang LS, Ruan YM, et al. Asymmetric mating interactions drive widespread invasion and displacement in a whitefly. Science 2007; 318:1769 - 72; http://dx.doi.org/10.1126/science.1149887; PMID: 17991828
- Pan HP, Chu D, Ge DQ, Wang SL, Wu QJ, Xie W, et al. Further spread of and domination by Bemisia tabaci (Hemiptera: Aleyrodidae) biotype Q on field crops in China. J Econ Entomol 2011; 104:978 - 85; http://dx.doi.org/10.1603/EC11009; PMID: 21735919
- Baumann P. Biology bacteriocyte-associated endosymbionts of plant sap-sucking insects. Annu Rev Microbiol 2005; 59:155 - 89; http://dx.doi.org/10.1146/annurev.micro.59.030804.121041; PMID: 16153167
- Feldhaar H. Bacterial symbionts as mediators of ecologically important traits of insect hosts. Ecol Entomol 2011; 36:533 - 43; http://dx.doi.org/10.1111/j.1365-2311.2011.01318.x
- Zchori-Fein E, Brown JK. Diversity of prokaryotes associated with Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae). Ann Entomol Soc Am 2002; 95:711 - 8; http://dx.doi.org/10.1603/0013-8746(2002)095[0711:DOPAWB]2.0.CO;2
- Thao ML, Baumann P. Evolutionary relationships of primary prokaryotic endosymbionts of whiteflies and their hosts. Appl Environ Microbiol 2004; 70:3401 - 6; http://dx.doi.org/10.1128/AEM.70.6.3401-3406.2004; PMID: 15184137
- Weeks AR, Velten R, Stouthamer R. Incidence of a new sex-ratio-distorting endosymbiotic bacterium among arthropods. Proc Biol Sci 2003; 270:1857 - 65; http://dx.doi.org/10.1098/rspb.2003.2425; PMID: 12964989
- Everett KD, Thao M, Horn M, Dyszynski GE, Baumann P. Novel chlamydiae in whiteflies and scale insects: endosymbionts ‘Candidatus Fritschea bemisiae’ strain Falk and ‘Candidatus Fritschea eriococci’ strain Elm. Int J Syst Evol Microbiol 2005; 55:1581 - 7; http://dx.doi.org/10.1099/ijs.0.63454-0; PMID: 16014485
- Gottlieb Y, Ghanim M, Chiel E, Gerling D, Portnoy V, Steinberg S, et al. Identification and localization of a Rickettsia sp. in Bemisia tabaci (Homoptera: Aleyrodidae). Appl Environ Microbiol 2006; 72:3646 - 52; http://dx.doi.org/10.1128/AEM.72.5.3646-3652.2006; PMID: 16672513
- Himler AG, Adachi-Hagimori T, Bergen JE, Kozuch A, Kelly SE, Tabashnik BE, et al. Rapid spread of a bacterial symbiont in an invasive whitefly is driven by fitness benefits and female bias. Science 2011; 332:254 - 6; http://dx.doi.org/10.1126/science.1199410; PMID: 21474763
- Mahadav A, Gerling D, Gottlieb Y, Czosnek H, Ghanim M. Parasitization by the wasp Eretmocerus mundus induces transcription of genes related to immune response and symbiotic bacteria proliferation in the whitefly Bemisia tabaci.. BMC Genomics 2008; 9:342; http://dx.doi.org/10.1186/1471-2164-9-342; PMID: 18638407
- Kontsedalov S, Zchori-Fein E, Chiel E, Gottlieb Y, Inbar M, Ghanim M. The presence of Rickettsia is associated with increased susceptibility of Bemisia tabaci (Homoptera: Aleyrodidae) to insecticides. Pest Manag Sci 2008; 64:789 - 92; http://dx.doi.org/10.1002/ps.1595; PMID: 18432613
- Ghanim M, Kontsedalov S. Susceptibility to insecticides in the Q biotype of Bemisia tabaci is correlated with bacterial symbiont densities. Pest Manag Sci 2009; 65:939 - 42; http://dx.doi.org/10.1002/ps.1795; PMID: 19479746
- Brumin M, Kontsedalov S, Ghanim M. Rickettsia influences thermotolerance in the whitefly Bemisia tabaci B biotype. Insect Sci 2011; 18:57 - 66; http://dx.doi.org/10.1111/j.1744-7917.2010.01396.x
- Gottlieb Y, Zchori-Fein E, Mozes-Daube N, Kontsedalov S, Skaljac M, Brumin M, et al. The transmission efficiency of tomato yellow leaf curl virus by the whitefly Bemisia tabaci is correlated with the presence of a specific symbiotic bacterium species. J Virol 2010; 84:9310 - 7; http://dx.doi.org/10.1128/JVI.00423-10; PMID: 20631135
- Kondo N, Shimada M, Fukatsu T. Infection density of Wolbachia endosymbiont affected by co-infection and host genotype. Biol Lett 2005; 1:488 - 91; http://dx.doi.org/10.1098/rsbl.2005.0340; PMID: 17148240
- Pan HP, Chu D, Liu BM, Xie W, Wang SL, Wu QJ, et al. The relative amount of symbionts in insect hosts changes with host-plant adaptation and insecticide resistance. Environ Entomol 2013;
- Wang J, Bing XL, Li M, Ye GY, Liu SS. Infection of tobacco plants by a begomovirus improves nutritional assimilation by a whitefly. Entomol Exp Appl 2012; 144:191 - 01; http://dx.doi.org/10.1111/j.1570-7458.2012.01278.x
- Chu D, Wan FH, Zhang YJ, Brown JK. Change in the biotype composition of Bemisia tabaci in Shandong Province of China from 2005 to 2008. Environ Entomol 2010; 39:1028 - 36; http://dx.doi.org/10.1603/EN09161; PMID: 20550819
- Pan HP, Li XC, Zhang YJ. Sex affects the infection frequencies of symbionts in Bemisia tabaci.. Commun Integr Biol 2012; 5:337 - 9; http://dx.doi.org/10.4161/cib.20398; PMID: 23060956
- Chu D, Gao CS, De Barro P, Zhang YJ, Wan FH, Khan IA. Further insights into the strange role of bacterial endosymbionts in whitefly, Bemisia tabaci: comparison of secondary symbionts from biotypes B and Q in China. Bull Entomol Res 2011; 101:477 - 86; http://dx.doi.org/10.1017/S0007485311000083; PMID: 21329550
- Tsuchida T, Koga R, Fukatsu T. Host plant specialization governed by facultative symbiont. Science 2004; 303:1989; http://dx.doi.org/10.1126/science.1094611; PMID: 15044797
- Simon JC, Carré S, Boutin M, Prunier-Leterme N, Sabater-Mun B, Latorre A, et al. Host-based divergence in populations of the pea aphid: insights from nuclear markers and the prevalence of facultative symbionts. Proc Biol Sci 2003; 270:1703 - 12; http://dx.doi.org/10.1098/rspb.2003.2430; PMID: 12964998
- Toju H, Fukatsu T. Diversity and infection prevalence of endosymbionts in natural populations of the chestnut weevil: relevance of local climate and host plants. Mol Ecol 2011; 20:853 - 68; http://dx.doi.org/10.1111/j.1365-294X.2010.04980.x; PMID: 21199036
- Gueguen G, Vavre F, Gnankine O, Peterschmitt M, Charif D, Chiel E, et al. Endosymbiont metacommunities, mtDNA diversity and the evolution of the Bemisia tabaci (Hemiptera: Aleyrodidae) species complex. Mol Ecol 2010; 19:4365 - 76; http://dx.doi.org/10.1111/j.1365-294X.2010.04775.x; PMID: 20723069
- Santos-Garcia D, Farnier PA, Beitia F, Zchori-Fein E, Vavre F, Mouton L, et al. Complete genome sequence of “Candidatus Portiera aleyrodidarum” BT-QVLC, an obligate symbiont that supplies amino acids and carotenoids to Bemisia tabaci.. J Bacteriol 2012; 194:6654 - 5; http://dx.doi.org/10.1128/JB.01793-12; PMID: 23144402