Abstract
Dictyostelium discoideum cells respond to periodic signals of extracellular cAMP by collective changes of cell-cell and cell-substrate contacts. This was confirmed by dielectric analysis employing electric cell-substrate impedance sensing (ECIS) and impedance measurements involving cell-filled micro channels in conjunction with optical microscopy providing a comprehensive picture of chemotaxis under conditions of starvation.
Dictyostelium discoideum (D. discoideum) is an unicellular amoeba in the growth phase that transforms its life form during starvation into a multicellular organism.Citation1,Citation2 10,000–100,000 amoebae develop a fruit body with spores that are able to survive unfavorable conditions.Citation2 Aggregation is initiated by cell-cell communication through coordinated movement of the individual cells triggered by the chemoattractant cyclic adenosine 3′,5′-monophosphate (cAMP). Therefore, the chemotaxis of D. discoideum amoebae is regulated by the periodic and synchronous synthesis, secretion, and decomposition of cAMP by the cells, which serves as directional signal for migration.Citation3
The detection of a cAMP-signal by an amoeba causes further release of cAMP as signal transfer for the nearby cells on the one hand and a polarization of the cell with an extension of pseudopodia in direction of the highest cAMP concentration followed by directional migration in this direction for 2–3 min on the other hand.Citation4-Citation7 By spatiotemporal evolution of the cAMP wave, an emitting center is generated that later serves as an aggregation center.Citation8,Citation9 This coordinated and collective behavior of the amoebae can be visualized by dark-field microscopyCitation10,Citation11 or light scattering experiments of suspended D. discoideum cells.Citation12 The periodic production and release of cAMP can be detected by a fluorescence resonance energy transfer (FRET)-based sensor measuring the intracellular cAMP concentrationCitation8 and by cAMP-isotope dilution-fluorography, respectively.Citation13
Chemotaxis of D. discoideum has so far mainly been described by means of optical microscopy providing data on cell shape changes and the velocity of migrating amoebae.Citation10,Citation14 However, changes in cell-cell and cell-substrate interactions associated with cAMP oscillations have so far not been addressed.
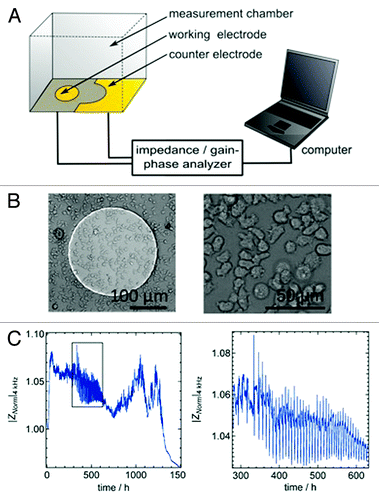
Recently, we established a non-optical method based on time-resolved impedance recordings to detect the collective behavior of a small ensemble of D. discoideum cells during the aggregation phase.Citation15,Citation16 The method referred to as electric cell-substrate impedance sensing (ECIS) essentially measures the dielectric characteristics of the cell bodies in contact with the electrode.Citation17 The impedance signal is caused by the cells adhering to the working electrode (d = 250 µm; d, diameter) that is located at the bottom of the culture dish serving as the measurement chamber (). In our previous publication entitled “Collective behavior of Dictyostelium discoideum monitored by impedance analysis,” we found that periodical changes of cell-cell as well as cell-substrate contacts largely explain impedance fluctuations during chemotaxis of amoebae.Citation15
Figure 1. (A) Schematic illustration of the experiment comprising electric cell-substrate impedance sensing (ECIS) setup mounted on top of an optical microscope. The complex impedance between the small working electrode and the large counter electrode is measured with an impedance analyzer (SI 1260). (B) Optical micrograph of a D. discoideum covered gold-electrode. (C) Magnitude of normalized impedance of an ECIS electrode measured at 4 kHz (|Znorm|4kHz) as a function of time. The black box highlights the time period during which collective oscillations occur due to starvation conditions (C, right graph).
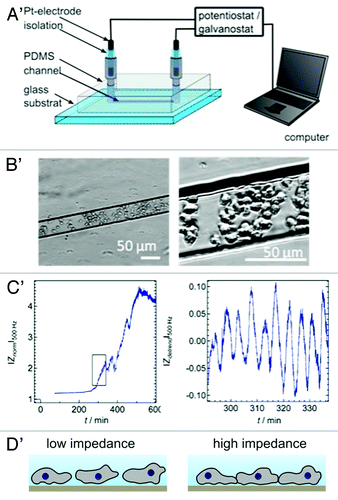
Additionally, we used a microfluidic device prepared by micromolding in capillaries, which allowed us to monitor temporal changes in impedance originating from D. discoideum cells filled into the PDMS (polydimethylsiloxane) micro channels (h = 18 µm, w = 50 µm, l = 5 mm; h, height; w, width; l, length) equipped with two blackened Pt-electrodes at both ends of the channel (). The setup is conceptionally based on an earlier publication of O’Connor et al.Citation18
Figure 2. (A) Microfluidic device prepared by micromolding in capillaries to monitor impedance fluctuations due to cell movements and shape changes within in the micro channel. The two entrances of the channel are equipped with two opposing electrodes. (B) The optical micrographs are taken with an inverted micrograph and show the amoebae dwelling in the channel. (C) Starvation triggers impedance oscillations in micro channel with a similar period as those found in ECIS measurements (blue lines). The zoom-in on the right side has been detrended to emphasize the impedance oscillations. (D) Scheme of the two proposed states that amoebae assume during impedance oscillations. At the impedance maxima the overall contact zone and the cell-cell contacts increase.
In both cases, ECIS and micro channel impedance measurements, after addition of starved cells in a glucose-free buffer the overall impedance increases and non-correlated impedance fluctuations appear caused by migration of the cells. After 3–5 h of cell starvation the impedance signal starts to display oscillations with a defined time period of a few minutes (5–12 min). This oscillation is caused by the characteristic 6 min cycles of cAMP-release and collective chemotaxis of D. discoideum during the aggregation phase. Albeit the biochemical origin of oscillations during chemotaxis is well-understood it remained to be elucidated what morphological changes of the cells are responsible for the observed impedance oscillations.
A number of potential contributions to the overall impedance might be envisioned to explain the periodic impedance spikes, for instance, shape/size changes of the cells, clustering and separation of the cells or variations in the cell-substrate distances. Through synchronic bright field (BF)-video recording of the cells on the working electrode during the ECIS measurements, it was possible to find reasons for the oscillation in the impedance signal. Analyzing the occupancy rate of the cells on the electrode does not show periodic changes in the total vertical size of the amoebae. Therefore, changes in the cell covered area do not cause the impedance oscillations. However, periodic changes between roundish and elongated cells are found by determining the circularity of the cells on the electrode over the time. We found that circularity displays the same frequency than impedance oscillation. Yet these changes in the shape of the cells cannot be the reason for the detected impedance spikes because of the phase shift between circularity and impedance signals and the different shape of the spikes.Citation15
Optical micrographs (BF-images) suggest that a collective process of reversible cell clustering occurs during chemotaxis. Counting the number of isolated D. discoideum amoebae displaying no shared cell-cell boundaries, a periodic formation of 2D clusters is detectable over the time. Each peak of maximum impedance corresponds to a local minimum in isolated cell number.Citation15 A simple equivalent circuit could show that even with a constant surface coverage larger isolating islands (more clustered cell distribution) produce a higher impedance signal than a homogeneous distribution with smaller islands (single, isolated cell distribution). Therefore, one possible reason for the oscillating impedance signal is periodical changes in lateral cell-cell organization at otherwise constant overall electrode coverage with amoebae.Citation15
Apart from reversible 2D cluster formation another reason for the occurrence of spikes in the impedance signal was identified and this is temporal and synchronous changes of the distance between cells and substrate.Citation15 Closer distances of the cells to the electrode produce larger impedance since the ionic flux underneath the cells is restricted to a smaller volume. For analyzing the time dependent variation in cell-substrate distance we used two different methods, quartz crystal microbalance measurements and TIRF (total internal reflection fluorescence) microscopy. Quartz crystal microbalance with dissipation monitoring (D-QCM) displays variations in cell-substrate distance if dissipation signal and resonance frequency shifts of the acoustic resonator are anti-correlated.Citation19 The recorded antidromic oscillation of dissipation and resonance frequency with a time period of 6 min some hours after starvation is a clear sign of cells periodically approaching toward and retracting from the substrate in response to the cAMP release cycle.Citation15
The second method to analyze the cell-substrate distance was TIRF video microscopy with cytosolic GFP-labeled cells. The time dependent overall fluorescence intensity of the recorded images shows oscillation approximately 4 h after cell starvation. The variation of the fluorescence intensity is caused by changes of the detectable cell area in close distance to the surface and not due to changes of the minimal distance between cell and surface.Citation15
Unfortunately, simultaneous measurements of TIRF and ECIS impedance data are not possible due to the insufficiently transparent gold electrode, which might also quench fluorescence. However, by comparison of simultaneously recorded BF images during ECIS measurements with quasi-simultaneously recorded BF images during recording of TIRF videos it was possible to indirectly correlate ECIS and TIRF time traces. As a consequence, we found that high impedance values correlate with the time point of high fluorescence intensity and therefore with a small overall cell-substrate distance. This makes perfectly sense since impedance increases with decreasing cell-substrate distance.Citation15 Impedance fluctuations in micro channels are also attributed to changes in 2D clustering and shape changes as well as periodic cell-substrate interactions. Future experiments with simultaneous optical imaging will be necessary to tell apart the different contributions.
Taken together, we conclude that impedance measurements in micro channels and on small electrodes are suitable methods to analyze collective behavior of cells in a label-free and non-invasive manner. The detected oscillation in the ECIS impedance signal can be explained by periodic changes of cell-substrate distance and changes in cell aggregation by reversible forming of cell-cell contacts during the chemotaxis of D. discoideum. Due to the similar signal shape and the high signal-to-noise ratio of the TIRF and the QCM measurements that are comparable with the ECIS signal, the dominant source of the observed impedance oscillation in ECIS measurements is probably the periodic change of the overall cell-substrate distance.
Acknowledgments
The AX2 HG1694 cell line (D. discoideum) was a friendly gift of Günther Gerisch, Max Planck Institute for Biochemistry; Martinsried, Germany. We also gratefully acknowledge financial support from the DFG through SFB 937 (“Collective behavior of soft and biological matter”).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Kessin RH. Dictyostelium Evolution, Cell Biology, and the Development of Multicellularity. Cambride University Press 2001; 1
- Dormann D, Vasiev B, Weijer CJ. Propagating waves control Dictyostelium discoideum morphogenesis. Biophys Chem 1998; 72:21 - 35; http://dx.doi.org/10.1016/S0301-4622(98)00120-3; PMID: 9652084
- Van Haastert PJM, Devreotes PN. Chemotaxis: signalling the way forward. Nat Rev Mol Cell Biol 2004; 5:626 - 34; http://dx.doi.org/10.1038/nrm1435; PMID: 15366706
- Kimmel AR, Parent CA. The signal to move: D. discoideum go orienteering. Science 2003; 300:1525 - 7; http://dx.doi.org/10.1126/science.1085439; PMID: 12791977
- Swaney KF, Huang C-H, Devreotes PN. Eukaryotic chemotaxis: a network of signaling pathways controls motility, directional sensing, and polarity. Annu Rev Biophys 2010; 39:265 - 89; http://dx.doi.org/10.1146/annurev.biophys.093008.131228; PMID: 20192768
- Tani T, Naitoh Y. Chemotactic responses of Dictyostelium discoideum amoebae to a cyclic AMP concentration gradient: evidence to support a spatial mechanism for sensing cyclic AMP. J Exp Biol 1999; 202:1 - 12; PMID: 9841889
- Weijer CJ. Dictyostelium morphogenesis. Curr Opin Genet Dev 2004; 14:392 - 8; http://dx.doi.org/10.1016/j.gde.2004.06.006; PMID: 15261655
- Gregor T, Fujimoto K, Masaki N, Sawai S. The onset of collective behavior in social amoebae. Science 2010; 328:1021 - 5; http://dx.doi.org/10.1126/science.1183415; PMID: 20413456
- Wang CJ, Bergmann A, Lin B, Kim K, Levchenko A. Diverse sensitivity thresholds in dynamic signaling responses by social amoebae. Sci Signal 2012; 5:ra17; http://dx.doi.org/10.1126/scisignal.2002449; PMID: 22375055
- Alcantara F, Monk M. Signal propagation during aggregation in the slime mould Dictyostelium discoideum.. J Gen Microbiol 1974; 85:321 - 34; http://dx.doi.org/10.1099/00221287-85-2-321; PMID: 4615133
- Sawai S, Thomason PA, Cox EC. An autoregulatory circuit for long-range self-organization in Dictyostelium cell populations. Nature 2005; 433:323 - 6; http://dx.doi.org/10.1038/nature03228; PMID: 15662425
- Gerisch G, Hess B. Cyclic-AMP-controlled oscillations in suspended Dictyostelium cells: their relation to morphogenetic cell interactions. Proc Natl Acad Sci USA 1974; 71:2118 - 22; http://dx.doi.org/10.1073/pnas.71.5.2118; PMID: 4365764
- Tomchik KJ, Devreotes PN. Adenosine 3′,5′-monophosphate waves in Dictyostelium discoideum: a demonstration by isotope dilution--fluorography. Science 1981; 212:443 - 6; http://dx.doi.org/10.1126/science.6259734; PMID: 6259734
- McCann CP, Kriebel PW, Parent CA, Losert W. Cell speed, persistence and information transmission during signal relay and collective migration. J Cell Sci 2010; 123:1724 - 31; http://dx.doi.org/10.1242/jcs.060137; PMID: 20427323
- Schäfer E, Tarantola M, Polo E, Westendorf C, Oikawa N, Bodenschatz E, et al. Chemotaxis of Dictyostelium discoideum: collective oscillation of cellular contacts. PLoS ONE 2013; 8:e54172; http://dx.doi.org/10.1371/journal.pone.0054172; PMID: 23349816
- Schäfer E, Westendorf C, Bodenschatz E, Beta C, Geil B, Janshoff A. Shape oscillations of Dictyostelium discoideum cells on ultramicroelectrodes monitored by impedance analysis. Small 2011; 7:723 - 6; http://dx.doi.org/10.1002/smll.201001955; PMID: 21425455
- Wegener J, Keese CR, Giaever I. Electric cell-substrate impedance sensing (ECIS) as a noninvasive means to monitor the kinetics of cell spreading to artificial surfaces. Exp Cell Res 2000; 259:158 - 66; http://dx.doi.org/10.1006/excr.2000.4919; PMID: 10942588
- O’Connor ER, Kimelberg HK, Keese CR, Giaever I. Electrical resistance method for measuring volume changes in monolayer cultures applied to primary astrocyte cultures. Am J Physiol 1993; 264:C471 - 8; PMID: 8447377
- Tarantola M, Pietuch A, Schneider D, Rother J, Sunnick E, Rosman C, et al. Toxicity of gold-nanoparticles: synergistic effects of shape and surface functionalization on micromotility of epithelial cells. Nanotoxicology 2011; 5:254 - 68; http://dx.doi.org/10.3109/17435390.2010.528847; PMID: 21050076