Abstract
SIMPLE, also known as LITAF, EET1 and PIG7, was originally identified based on its transcriptional upregulation by estrogen, p53, lipopolysaccharide or a microbial cell-wall component. Missense mutations in SIMPLE cause Charcot-Marie-Tooth disease (CMT), and altered SIMPLE expression is associated with cancer, obesity and inflammatory bowel diseases. Despite increasing evidence linking SIMPLE to human diseases, the biological function of SIMPLE is unknown and the pathogenic mechanism of SIMPLE mutations remains elusive. Our recent study reveals that SIMPLE is a functional partner of the endosomal sorting complex required for transport (ESCRT) machinery in the regulation of endosome-to-lysosome trafficking and intracellular signaling. Our results indicate that CMT-linked SIMPLE mutants are loss-of-function mutants which act dominantly to impair endosomal trafficking and signaling attenuation. We propose that endosomal trafficking and signaling dysregulation is a key pathogenic mechanism in CMT and other diseases that involve SIMPLE dysfunction.
Charcot-Marie-Tooth disease (CMT) is the most common hereditary peripheral neuropathy with no effective treatment.Citation1 Human genetic studies reveal that missense mutations in SIMPLE, a protein of unknown function, cause autosomal dominant CMT type 1C (CMT1C).Citation2-Citation6 SIMPLE, also known as LITAF, EET1 and PIG7, was originally identified as a gene product whose transcription is upregulated by estrogen,Citation7 p53 protein,Citation8 lipopolysaccharide (LPS)Citation9 and a microbial cell-wall component.Citation10 Reduced SIMPLE expression is associated with several types of cancer, including breast cancer,Citation11 lymphoma,Citation12 leukemiaCitation13 and thyroid carcinoma,Citation14 whereas increased SIMPLE expression is linked to obesityCitation15 and inflammatory bowel diseases, such as Crohn’s disease and ulcerative colitis.Citation16 The connection of SIMPLE to multiple human diseases underscores the importance of understanding the biochemical function and cellular role of this enigmatic protein.
SIMPLE Functions in the Regulation of Endosome-to-Lysosome Trafficking and Cell Signaling
Endosome-to-lysosome trafficking is a crucial cellular process that not only controls protein degradation but also regulates intracellular signaling.Citation17,Citation18 Receptors at cell surface are internalized in response to ligand binding and delivered to the early endosome, where they are either recycled to the cell surface or sorted to intralumenal vesicles of multivesicular bodies for transport to the lysosome for degradation. The core machinery for mediating endosomal cargo sorting to the lysosomal pathway comprises ESCRT-0, -I, -II and -III complexes.Citation17 The ESCRT accessory factors and mechanisms that confer temporal and spatial control to the endosomal trafficking process remain largely unknown.
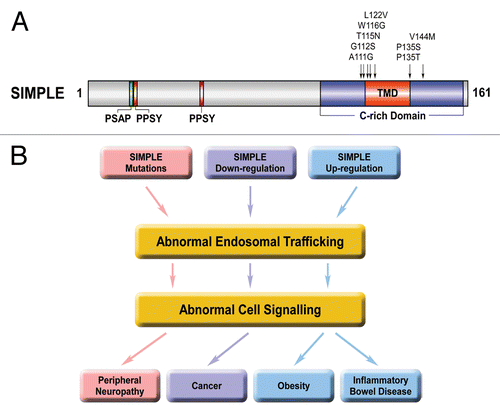
SIMPLE is a 161-amino-acid protein with widespread expression in a variety of tissues and cells.Citation2,Citation10,Citation19,Citation20 Although a distinct transcript coding for a 228-amino-acid protein was reported to be encoded by the SIMPLE gene,Citation9 it is now clear that this larger transcript is the result of a DNA sequencing error.Citation4,Citation10,Citation21 Accumulating evidenceCitation4,Citation10,Citation21 indicates that SIMPLE is unlikely to be a transcription factor as initially proposed.Citation9 The function of SIMPLE is unknown, although it contains binding sites for TSG101 and NEDD4 ().Citation22 SIMPLE also contains a cysteine-rich (C-rich) domain () that was hypothesized to be a putative RING finger with E3 ubiquitin-protein ligase activity.Citation10,Citation23 However, our analysis shows that the SIMPLE C-rich domain is not a RING finger because it lacks a key His residue and is interrupted by an embedded transmembrane domain ().Citation19 Furthermore, the results of our biochemical experiments reveal that SIMPLE protein has no E3 ligase activity either in vitro or in vivo.Citation24 By using highly specific anti-SIMPLE antibodies, we found that endogenous SIMPLE is an early endosomal membrane protein,Citation19 rather than a nuclear proteinCitation9,Citation25 or a lysosomal/late endosomal membrane protein,Citation10 as previously suggested.
Figure 1. Endosomal trafficking and signaling dysregulation as a potential pathogenic mechanism in CMT and other diseases that involve SIMPLE dysfunction. (A) Domain structure of SIMPLE and mutations found in CMT1C patients. PSAP, predicted TSG101-binding site; PPSY, predicted NEDD4-binding site; C-rich domain, cysteine-rich domain; TMD, predicted transmembrane domain. The locations of CMT1C-linked SIMPLE mutations are indicated on the domain structure. (B) Potential pathogenic roles of SIMPLE dysfunction in CMT, cancer, obesity and inflammatory bowel diseases. Our recent workCitation24 suggests a pathogenic pathway by which CMT1C-linked SIMPLE mutationsCitation2-Citation6 cause demyelinating peripheral neuropathy by disrupting endosome-to-lysosome trafficking and signaling attenuation of NRG1-activated ErbB2/ErbB3 receptors and consequently prolonging their signaling to downstream pathways in Schwann cells (colored in pink). Downregulation of SIMPLE expression found in several types of cancerCitation11-Citation14 may contribute to the process of malignant transformation by impairing endosome-to-lysosome trafficking and signaling attenuation of mitogenic signaling receptors (colored in lavender). Upregulation of SIMPLE expression found in obesityCitation15 and inflammatory bowel diseasesCitation16 may contribute to the pathogenesis or progression of these diseases by altering endosomal trafficking and intracellular signaling (colored in blue).
In a recent study,Citation24 we examined the cellular function of SIMPLE and found that SIMPLE is a novel regulator of endosome-to-lysosome trafficking. Our results indicate that SIMPLE participates in the recruitment of ESCRT components STAM1, Hrs and TSG101 to the early endosomal membrane and functions with the ESCRT machinery in controlling endosomal sorting and lysosomal degradation of cargo proteins, such as ErbB receptors. In addition, we found that SIMPLE is required for efficient attenuation of signaling events downstream of ligand-activated ErbB receptors. Our data support that SIMPLE regulates cell signaling by promoting endosome-to-lysosome trafficking and degradation of signaling receptors.Citation24 Given its widespread expression pattern,Citation2,Citation10,Citation19,Citation20 our findings suggest that SIMPLE may regulate endosomal trafficking and signaling processes in many different cells, including LPS-induced inflammatory signaling in macrophages.Citation26
Endosomal Trafficking and Signaling Dysregulation: A Key Mechanism in CMT Pathogenesis
Despite the identification of eight distinct point mutations in SIMPLE () as the genetic defects for causing CMT1C,Citation2-Citation6 the pathogenic mechanisms of these mutations are unknown. We have shown that SIMPLE is a post-translationally inserted, C-tail-anchored membrane protein that uses its TMD for anchoring to the early endosomal membrane.Citation19,Citation24 Interestingly, all of the identified disease-causing SIMPLE mutations map in and around the TMD (). We found that CMT1C-linked SIMPLE W116G and P135T mutations promote SIMPLE misfolding and impair its membrane insertion, causing SIMPLE to mislocalize from the endosomal membrane to the cytosol.Citation19,Citation27 Our recent results indicate that SIMPLE W116G and P135T are loss-of-function mutants that exert dominant pathogenic effects to impair endosome-to-lysosome trafficking and signaling attenuation in cells.Citation24 The dominant pathogenic role of SIMPLE mutation is further supported by our finding of a CMT1C-like peripheral neuropathy phenotype in transgenic mice expressing SIMPLE W116G mutantCitation28 and the lack of a neuropathy phenotype in SIMPLE knockout mice.Citation20
Our work reveals a critical role of dysregulated endosome-to-lysosome trafficking in the pathogenesis of demyelinating CMT1C.Citation24 Previous studies have shown that mutations in MTMR2 and MTMR13, which are also involved in regulation of endosomal trafficking, cause demyelinating CMT4B1 and CMT4B2.Citation1,Citation29 Thus, endosomal trafficking dysregulation appears to be a common pathogenic mechanism in several demyelinating CMT diseases. The fact that mutations in these ubiquitously expressed proteins cause demyelinating peripheral neuropathy suggests that, compared with other cell types, Schwann cells are particularly susceptible to defects in endosomal trafficking. We found that SIMPLE is highly abundant in Schwann cellsCitation19,Citation28 and that SIMPLE W116G and P135T mutations cause dysregulation of neuregulin-1 (NRG1)-ErbB2/ErbB3 signaling,Citation24 a key pathway for controlling peripheral nerve myelination by Schwann cells.Citation30 In our transgenic CMT1C mouse model, SIMPLE W116G mutation-induced peripheral neuropathy is accompanied by myelin infolding—the focally infolded myelin loops that protrude into the axons.Citation28 Together, our findings suggest a pathogenic pathway () by which SIMPLE mutation disrupts endosome-to-lysosome trafficking and signaling attenuation of NRG1-activated ErbB2/ErbB3 receptors in Schwann cells, causing prolonged activation of downstream signaling pathways, thereby leading to myelin infolding and demyelinating peripheral neuropathy.
Conclusions
Our recent study revealing the function of SIMPLE as a regulator of endosome-to-lysosome trafficking and intracellular signaling has provided new insights into the mechanisms of SIMPLE action in health and disease. The evidence obtained from our work indicates that SIMPLE mutation-induced endosomal trafficking and signaling dysregulation in Schwann cells play a key role in CMT1C pathogenesis. Our findings also raised the possibility that altered SIMPLE expression found in cancer,Citation11-Citation14 obesityCitation15 and inflammatory bowel diseasesCitation16 may contribute to the pathogenesis or progression of these diseases by altering SIMPLE-dependent endosomal trafficking and signaling (). Future studies to examine this possibility and identify trafficking and signaling defects caused by SIMPLE dysfunction should enhance our understanding of the pathogenic mechanisms involved in CMT and other diseases and may provide new strategies for therapeutic intervention.
| Abbreviations: | ||
| CMT | = | Charcot-Marie-Tooth disease |
| CMT1C | = | CMT type 1C |
| C-rich | = | cysteine-rich |
| ESCRT | = | endosomal sorting complex required for transport |
| NRG1 | = | neuregulin-1 |
| TMD | = | transmembrane domain |
Acknowledgments
This work was supported in part by the National Institutes of Health (AG034126 to L.S.C. and ES015813 and GM103613 to L.L.).
References
- Patzkó A, Shy ME. Update on Charcot-Marie-Tooth disease. Curr Neurol Neurosci Rep 2011; 11:78 - 88; http://dx.doi.org/10.1007/s11910-010-0158-7; PMID: 21080241
- Street VA, Bennett CL, Goldy JD, Shirk AJ, Kleopa KA, Tempel BL, et al. Mutation of a putative protein degradation gene LITAF/SIMPLE in Charcot-Marie-Tooth disease 1C. Neurology 2003; 60:22 - 6; http://dx.doi.org/10.1212/WNL.60.1.22; PMID: 12525712
- Bennett CL, Shirk AJ, Huynh HM, Street VA, Nelis E, Van Maldergem L, et al. SIMPLE mutation in demyelinating neuropathy and distribution in sciatic nerve. Ann Neurol 2004; 55:713 - 20; http://dx.doi.org/10.1002/ana.20094; PMID: 15122712
- Saifi GM, Szigeti K, Wiszniewski W, Shy ME, Krajewski K, Hausmanowa-Petrusewicz I, et al. SIMPLE mutations in Charcot-Marie-Tooth disease and the potential role of its protein product in protein degradation. Hum Mutat 2005; 25:372 - 83; http://dx.doi.org/10.1002/humu.20153; PMID: 15776429
- Latour P, Gonnaud PM, Ollagnon E, Chan V, Perelman S, Stojkovic T, et al. SIMPLE mutation analysis in dominant demyelinating Charcot-Marie-Tooth disease: three novel mutations. J Peripher Nerv Syst 2006; 11:148 - 55; http://dx.doi.org/10.1111/j.1085-9489.2006.00080.x; PMID: 16787513
- Gerding WM, Koetting J, Epplen JT, Neusch C. Hereditary motor and sensory neuropathy caused by a novel mutation in LITAF. Neuromuscul Disord 2009; 19:701 - 3; http://dx.doi.org/10.1016/j.nmd.2009.05.006; PMID: 19541485
- Everett LM, Li A, Devaraju G, Caperell-Grant A, Bigsby RM. A novel estrogen-enhanced transcript identified in the rat uterus by differential display. Endocrinology 1997; 138:3836 - 41; http://dx.doi.org/10.1210/en.138.9.3836; PMID: 9275072
- Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B. A model for p53-induced apoptosis. Nature 1997; 389:300 - 5; http://dx.doi.org/10.1038/38525; PMID: 9305847
- Myokai F, Takashiba S, Lebo R, Amar S. A novel lipopolysaccharide-induced transcription factor regulating tumor necrosis factor alpha gene expression: molecular cloning, sequencing, characterization, and chromosomal assignment. Proc Natl Acad Sci USA 1999; 96:4518 - 23; http://dx.doi.org/10.1073/pnas.96.8.4518; PMID: 10200294
- Moriwaki Y, Begum NA, Kobayashi M, Matsumoto M, Toyoshima K, Seya T. Mycobacterium bovis Bacillus Calmette-Guerin and its cell wall complex induce a novel lysosomal membrane protein, SIMPLE, that bridges the missing link between lipopolysaccharide and p53-inducible gene, LITAF(PIG7), and estrogen-inducible gene, EET-1. J Biol Chem 2001; 276:23065 - 76; http://dx.doi.org/10.1074/jbc.M011660200; PMID: 11274176
- Abba MC, Drake JA, Hawkins KA, Hu Y, Sun H, Notcovich C, et al. Transcriptomic changes in human breast cancer progression as determined by serial analysis of gene expression. Breast Cancer Res 2004; 6:R499 - 513; http://dx.doi.org/10.1186/bcr899; PMID: 15318932
- Mestre-Escorihuela C, Rubio-Moscardo F, Richter JA, Siebert R, Climent J, Fresquet V, et al. Homozygous deletions localize novel tumor suppressor genes in B-cell lymphomas. Blood 2007; 109:271 - 80; http://dx.doi.org/10.1182/blood-2006-06-026500; PMID: 16960149
- Wang D, Liu J, Tang K, Xu Z, Xiong X, Rao Q, et al. Expression of pig7 gene in acute leukemia and its potential to modulate the chemosensitivity of leukemic cells. Leuk Res 2009; 33:28 - 38; http://dx.doi.org/10.1016/j.leukres.2008.06.034; PMID: 18674816
- Lui WO, Foukakis T, Lidén J, Thoppe SR, Dwight T, Höög A, et al. Expression profiling reveals a distinct transcription signature in follicular thyroid carcinomas with a PAX8-PPAR(gamma) fusion oncogene. Oncogene 2005; 24:1467 - 76; http://dx.doi.org/10.1038/sj.onc.1208135; PMID: 15608688
- Ji ZZ, Dai Z, Xu YC. A new tumor necrosis factor (TNF)-α regulator, lipopolysaccharides-induced TNF-α factor, is associated with obesity and insulin resistance. Chin Med J (Engl) 2011; 124:177 - 82; PMID: 21362361
- Stucchi A, Reed K, O’Brien M, Cerda S, Andrews C, Gower A, et al. A new transcription factor that regulates TNF-alpha gene expression, LITAF, is increased in intestinal tissues from patients with CD and UC. Inflamm Bowel Dis 2006; 12:581 - 7; http://dx.doi.org/10.1097/01.MIB.0000225338.14356.d5; PMID: 16804395
- Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev Cell 2011; 21:77 - 91; http://dx.doi.org/10.1016/j.devcel.2011.05.015; PMID: 21763610
- Wegner CS, Rodahl LM, Stenmark H. ESCRT proteins and cell signalling. Traffic 2011; 12:1291 - 7; http://dx.doi.org/10.1111/j.1600-0854.2011.01210.x; PMID: 21518165
- Lee SM, Olzmann JA, Chin LS, Li L. Mutations associated with Charcot-Marie-Tooth disease cause SIMPLE protein mislocalization and degradation by the proteasome and aggresome-autophagy pathways. J Cell Sci 2011; 124:3319 - 31; http://dx.doi.org/10.1242/jcs.087114; PMID: 21896645
- Somandin C, Gerber D, Pereira JA, Horn M, Suter U. LITAF (SIMPLE) regulates Wallerian degeneration after injury but is not essential for peripheral nerve development and maintenance: implications for Charcot-Marie-Tooth disease. Glia 2012; 60:1518 - 28; http://dx.doi.org/10.1002/glia.22371; PMID: 22729949
- Huang Y, Bennett CL. Litaf/Simple protein is increased in intestinal tissues from patients with CD and UC, but is unlikely to function as a transcription factor. Inflamm Bowel Dis 2007; 13:120 - 1; http://dx.doi.org/10.1002/ibd.20010; PMID: 17206649
- Shirk AJ, Anderson SK, Hashemi SH, Chance PF, Bennett CL. SIMPLE interacts with NEDD4 and TSG101: evidence for a role in lysosomal sorting and implications for Charcot-Marie-Tooth disease. J Neurosci Res 2005; 82:43 - 50; http://dx.doi.org/10.1002/jnr.20628; PMID: 16118794
- Berger P, Niemann A, Suter U. Schwann cells and the pathogenesis of inherited motor and sensory neuropathies (Charcot-Marie-Tooth disease). Glia 2006; 54:243 - 57; http://dx.doi.org/10.1002/glia.20386; PMID: 16856148
- Lee SM, Chin LS, Li L. Charcot-Marie-Tooth disease-linked protein SIMPLE functions with the ESCRT machinery in endosomal trafficking. J Cell Biol 2012; 199:799 - 816; http://dx.doi.org/10.1083/jcb.201204137; PMID: 23166352
- Tang X, Fenton MJ, Amar S. Identification and functional characterization of a novel binding site on TNF-alpha promoter. Proc Natl Acad Sci USA 2003; 100:4096 - 101; http://dx.doi.org/10.1073/pnas.0630562100; PMID: 12655064
- Tang X, Metzger D, Leeman S, Amar S. LPS-induced TNF-alpha factor (LITAF)-deficient mice express reduced LPS-induced cytokine: Evidence for LITAF-dependent LPS signaling pathways. Proc Natl Acad Sci USA 2006; 103:13777 - 82; http://dx.doi.org/10.1073/pnas.0605988103; PMID: 16954198
- Lee SM, Chin LS, Li L. Protein misfolding and clearance in demyelinating peripheral neuropathies: Therapeutic implications. Commun Integr Biol 2012; 5:107 - 10; http://dx.doi.org/10.4161/cib.18638; PMID: 22482025
- Lee SM, Sha D, Mohammed AA, Asress S, Glass JD, Chin LS, et al. Motor and sensory neuropathy due to myelin infolding and paranodal damage in a transgenic mouse model of Charcot-Marie-Tooth disease type 1C. Hum Mol Genet 2013; In press http://dx.doi.org/10.1093/hmg/ddt022; PMID: 23359569
- Lee SM, Chin LS, Li L. Therapeutic implications of protein homeostasis in demyelinating peripheral neuropathies. Expert Rev Neurother 2012; 12:1041 - 3; http://dx.doi.org/10.1586/ern.12.79; PMID: 23039381
- Quintes S, Goebbels S, Saher G, Schwab MH, Nave KA. Neuron-glia signaling and the protection of axon function by Schwann cells. J Peripher Nerv Syst 2010; 15:10 - 6; http://dx.doi.org/10.1111/j.1529-8027.2010.00247.x; PMID: 20433601