Abstract
The retromer complex mediates endosomal protein sorting by concentrating membrane proteins (cargo) into nascent tubules formed through the action of sorting nexin (SNX) proteins. The WASH complex is recruited to endosomes by binding to the VPS35 subunit of retromer and facilitates cargo protein sorting by promoting formation of endosomally-localized F-actin. The VPS35 protein is mutated in Parkinson disease (PD) and a recent report has revealed that the PD-causing mutation impairs the association of retromer with the WASH complex leading to perturbed endosomal protein sorting. Another important player in endosomal protein sorting is the DNAJC13/RME-8 protein, which associates with SNX1 and has also recently been linked to PD. An additional recent report has now shown that RME-8 also interacts with the WASH complex thus establishing retromer and WASH complex-mediated endosomal protein sorting as a key pathway linked to the pathology of PD and providing new avenues to explore in the search for insights into the disease mechanism.
The retromer complex—a vital component of endosomal sorting machinery
Within eukaryotic cells, endosomes are key sorting stations that receive material (proteins and lipids) from both the biosynthetic and the endocytic pathways. A membrane protein arriving at an endosome can be sorted for delivery to one of three destinations: the cell surface, the Golgi, and the lysosome. The retromer complex is a conserved multimeric protein complex that is required to sort proteins into both the endosome-to-Golgi, and endosome-to-cell surface pathway. The role of cargo selection within retromer is performed by a trimer of the VPS35, VPS26, VPS29 proteins where binding to cargo is known to be mediated by both VPS35 and VPS26.Citation1 The other key activity of retromer is membrane bending to form tubules into which cargo is sorted. Tubules are formed by the action of the sorting nexin (SNX) dimer component of retromer, which associates loosely with the trimeric cargo-selective complex (CSC). The SNX dimer binds to phosphatidyl inositol 3-phosphate (PtdIns 3P) in the membrane and is able to generate tubules through an intrinsic self-assembly activity and the action of C-terminal Bin-Amphiphysin-Rvs (BAR) domains that adopt a curved banana-shaped conformation to drive tubulation.Citation2 The SNX1 protein is a key element of retromer-mediated endosomal protein sorting and can generate membrane tubules both in vivo and in vitro.Citation3
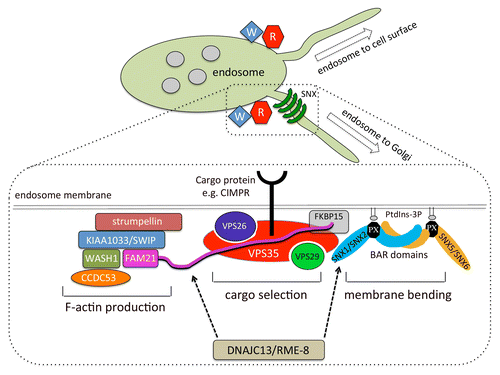
In addition to mediating sorting of membrane proteins into nascent tubular domains for delivery to the Golgi or the cell surface, the retromer CSC also functions as a hub for recruiting a host of accessory proteins to endosomes.Citation4 A key retromer accessory is the WASH complex—a pentameric protein complex that mediates production of filamentous (F-) actin patches on endosomes to facilitate the partitioning of membrane protein into discrete regions of the endosome so that sorting into distinct pathways is accomplished. Loss of WASH complex function results in an accumulation of endosomal tubules indicating that the WASH complex contributes to the scission of tubules, possibly through an association with Dynamin-2.Citation5 The recruitment of the WASH complex to endosomes occurs through the interaction of the VPS35 protein with the extended “tail” domain of the FAM21 protein of the WASH complex.Citation6-Citation8 Along with binding to the WASH complex, VPS35 also interacts with a protein called FKBP15 (also known as FKBP133 and WAFL). Thus a tripartite complex comprising the retromer CSC, the WASH complex, and FKBP15 is formed. The precise role of FKBP15 has yet to be determined but it may function to modulate actin dynamics at the endosome.Citation4,Citation6,Citation9 The components of the retromer and WASH complexes are shown schematically in .
Figure 1. Schematic diagram of the retromer and WASH complexes. The retromer cargo-selective complex (CSC) associates with endosomal membranes to sort cargo proteins (e.g. the cation-independent mannose 6-phosphate receptor—CIMPR) into tubules formed by the sorting nexin (SNX) dimer. The retromer CSC also recruits the WASH complex that mediates F-actin production through VPS35 binding to the extended tail of the FAM21 protein (shown in pink). The FKBP15 protein binds to both VPS35 and FAM21. The RME-8 protein has recently been shown to associate with the WASH complex through binding to FAM21 and regulates the kinetics of SNX dimer association-dissociation with the membrane.
RME-8, an accessory of the WASH complex and SNX dimer
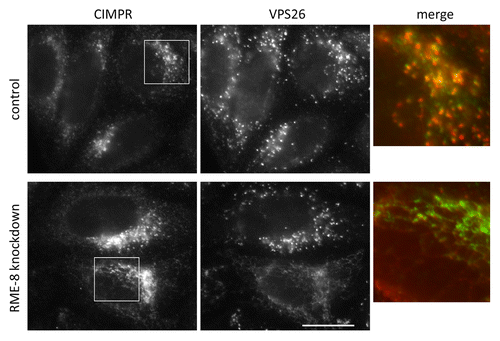
The sorting nexin dimer, including the SNX1 protein, also associates with accessory factors; one of which is a protein called RME-8—a member of the DNAJ family of proteins that are implicated in regulating the assembly and disassembly of macromolecular complexes via an interaction with chaperone proteins.Citation10 In a recent publication, it has been shown that RME-8 also associates with the WASH complex through binding to the tail of the FAM21 protein.Citation11 When RME-8 expression was abolished using RNAi, the morphology of endosomes was profoundly altered becoming a highly branched network of membrane tubules that contained many membrane proteins that require retromer and the WASH complex for their proper localization including the iron transporter protein SLC11a2 (also known as DMT1-II) and the α5β1 integrin complex. Proteins that comprise the retromer CSC, i.e., VPS26 and VPS35, also became extensively localized to tubules, as did the SNX1 protein.Citation11,Citation12 Endosomal tubulation observed after loss of RME-8 is shown in .
Figure 2. Loss of RME-8 function leads to extensive tubulation of endosomes. The expression of RME-8 was silenced using RNAi. After fixation and labeling with antibodies, cells were imaged using an epifluorescence microscope. The knockdown of RME-8 leads to an accumulation of endosomal tubules that are positive for retromer proteins and cargo such as the CIMPR. Scale bar = 20 μm.
It was hypothesised that the increase in membrane tubules observed after loss of RME-8 function may be due, at least in part, to reduced kinetics of SNX1 association and dissociation with the membrane. This was investigated using Fluorescence Recovery After Photobleaching (FRAP) experiments that confirmed that the kinetics of SNX1 association with the membrane were altered so that the recovery of GFP-SNX1 fluorescence was markedly slower after loss of RME-8 expression. The precise role of RME-8 in modulating the activity of the WASH complex has yet to be elucidated but it is possible that RME-8 functions to regulate the localization of the WASH complex so that the WASH complex is primarily localized to vesicular endosomes and not tubules as the function of the WASH complex to promote F-actin production is required on vesicular endosomes to facilitate sorting of membrane proteins. Consistent with this hypothesis, the FAM21 protein and a GFP-tagged WASH1 protein become localized to endosomal tubules following siRNA-mediated silencing of RME-8.Citation11
Parkinson disease-causing mutations to VPS35 and RME-8
Interestingly, the DNAJC13 gene that encodes RME-8 has recently been shown to be mutated in an inherited form of Parkinson disease (PD).Citation13 It is not currently known whether the PD-causing mutation affects the association of RME-8 with either the WASH complex or SNX1 but this may be a fruitful area to investigate. The identification of DNAJC13/RME-8 as a player in PD cements the importance of endosomal protein sorting in the pathogenesis of PD and follows the identification of VPS35 as a PD gene.Citation14-Citation16 For VPS35, the PD-causing mutation is a substitution of Aspartate for Asparagine at residue 620 (D620N). This mutation has now been shown to impair the association of the WASH complex with the retromer CSC resulting in reduced WASH complex recruitment to the endosome.Citation17 Thus the FAM21 protein of the WASH complex interacts with two proteins—VPS35 and RME-8—both of which are mutated in PD. This suggests that WASH complex function may be especially important in the mechanisms that underlie PD. In fact, WASH complex function is already associated with another neurodegenerative movement disorder, namely hereditary spastic paraplegia (HSP), as the strumpellin component of the WASH complex is encoded by the KIAA0196 gene at the SPG8 locus—a locus associated with HSP.Citation18 Currently it is not known why mutations in strumpellin cause HSP whilst mutations to WASH complex-associated proteins result in PD.
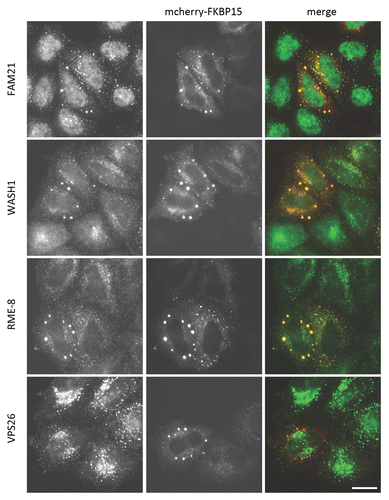
Curiously, although the retromer-WASH complex association is impaired by the VPS35 PD mutation, the most pronounced effect of the VPS35 D620N mutation is on the association with FKBP15, a protein whose role in endosomal protein sorting remains poorly understood.Citation17 One role of the FKBP15 protein may be to facilitate or stabilize the retromer-WASH complex association as increased FKBP15 expression can enable retromer to co-immunoprecipitate the WASH complex under less favourable conditions. Indeed when FKBP15 is overexpressed, in a subset of highly expressing cells, it forms cytoplasmic aggregates that contain both the WASH complex and RME-8 (see ) indicating that FKBP15 forms a strong association with the WASH complex and can exert dominant negative effects on WASH-complex associated proteins such as RME-8. The FKBP15 protein is linked to inflammatory bowel disease but it is currently not known what role FKBP15 plays in this disease.Citation19
Figure 3. FKBP15 is important for WASH complex localization. Cells were transiently transfected with mcherry-tagged FKBP15. In the highly expressing cells FKBP15 forms bright cytoplasmic aggregates that are also positive for WASH complex proteins and RME-8. The retromer protein VPS26 remains associated with endosomes however. Scale bar = 20 μm.
Among the PD-causing genes, mutations in LRRK2 are a frequent form of inherited PD.Citation20 It has recently been reported that overexpression of wildtype VPS35 could rescue lysosomal defects caused by LRRK2 mutants but the VPS35 D620N mutant does not rescue suggesting that retromer function is possibly downstream of LRRK2 activity.Citation21 Interestingly, it has been reported that the loss of LRRK2 function can also cause inflammatory bowel disease although it is mechanistically unclear how this occurs.Citation20,Citation22 As both LRRK2 and FKBP15 have been linked to inflammatory bowel disease and both are players in PD (FKBP15 through the VPS35 D620N mutant) it is tempting to speculate that a common mechanism or pathway involving FKBP15 and LRRK2 underlies both pathologies.
Conclusions
The retromer and WASH complexes play key roles in endosomal protein sorting and are emerging as cellular machinery that is especially important in many neurological diseases, including PD. The convergence of genetic studies identifying disease-causing mutations with the functional studies of endosomal protein sorting represents a significant advance in understanding the molecular basis of diseases such as PD, but there is much left to learn about how the various proteins operate in endosomal protein sorting and what consequences arise when these processes are perturbed. The next few years offer the potential for further advances that will illuminate both the pathology of PD and also the basic mechanisms of endosomal protein sorting.
| Abbreviations: | ||
| VPS | = | vacuolar protein sorting |
| CSC | = | cargo-selective complex |
| RME | = | receptor-mediated endocytosis |
| SNX | = | sorting nexin |
| PD | = | Parkinson disease |
| BAR | = | Bin/Amphiphysin/Rvs |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Seaman MN. The retromer complex - endosomal protein recycling and beyond. J Cell Sci 2012; 125:4693 - 702; http://dx.doi.org/10.1242/jcs.103440; PMID: 23148298
- van Weering JR, Sessions RB, Traer CJ, Kloer DP, Bhatia VK, Stamou D, Carlsson SR, Hurley JH, Cullen PJ. Molecular basis for SNX-BAR-mediated assembly of distinct endosomal sorting tubules. EMBO J 2012; 31:4466 - 80; http://dx.doi.org/10.1038/emboj.2012.283; PMID: 23085988
- Carlton J, Bujny M, Peter BJ, Oorschot VM, Rutherford A, Mellor H, Klumperman J, McMahon HT, Cullen PJ. Sorting nexin-1 mediates tubular endosome-to-TGN transport through coincidence sensing of high- curvature membranes and 3-phosphoinositides. Curr Biol 2004; 14:1791 - 800; http://dx.doi.org/10.1016/j.cub.2004.09.077; PMID: 15498486
- Harbour ME, Breusegem SY, Antrobus R, Freeman C, Reid E, Seaman MN. The cargo-selective retromer complex is a recruiting hub for protein complexes that regulate endosomal tubule dynamics. J Cell Sci 2010; 123:3703 - 17; http://dx.doi.org/10.1242/jcs.071472; PMID: 20923837
- Derivery E, Sousa C, Gautier JJ, Lombard B, Loew D, Gautreau A. The Arp2/3 activator WASH controls the fission of endosomes through a large multiprotein complex. Dev Cell 2009; 17:712 - 23; http://dx.doi.org/10.1016/j.devcel.2009.09.010; PMID: 19922875
- Harbour ME, Breusegem SY, Seaman MN. Recruitment of the endosomal WASH complex is mediated by the extended ‘tail’ of Fam21 binding to the retromer protein Vps35. Biochem J 2012; 442:209 - 20; http://dx.doi.org/10.1042/BJ20111761; PMID: 22070227
- Jia D, Gomez TS, Billadeau DD, Rosen MK. Multiple repeat elements within the FAM21 tail link the WASH actin regulatory complex to the retromer. Mol Biol Cell 2012; 23:2352 - 61; http://dx.doi.org/10.1091/mbc.E11-12-1059; PMID: 22513087
- Helfer E, Harbour ME, Henriot V, Lakisic G, Sousa-Blin C, Volceanov L, Seaman MN, Gautreau A. Endosomal recruitment of the WASH complex: active sequences and mutations impairing interaction with the retromer. Biol Cell 2013; 105:191 - 207; http://dx.doi.org/10.1111/boc.201200038; PMID: 23331060
- Viklund IM, Aspenström P, Meas-Yedid V, Zhang B, Kopec J, Agren D, Schneider G, D’Amato M, Olivo-Marin JC, Sansonetti P, et al. WAFL, a new protein involved in regulation of early endocytic transport at the intersection of actin and microtubule dynamics. Exp Cell Res 2009; 315:1040 - 52; http://dx.doi.org/10.1016/j.yexcr.2008.12.004; PMID: 19121306
- Shi A, Sun L, Banerjee R, Tobin M, Zhang Y, Grant BD. Regulation of endosomal clathrin and retromer-mediated endosome to Golgi retrograde transport by the J-domain protein RME-8. EMBO J 2009; 28:3290 - 302; http://dx.doi.org/10.1038/emboj.2009.272; PMID: 19763082
- Freeman CL, Hesketh G, Seaman MN. RME-8 coordinates the activity of the WASH complex with the function of the retromer SNX dimer to control endosomal tubulation. J Cell Sci 2014; 127:2053 - 70; http://dx.doi.org/10.1242/jcs.144659; PMID: 24643499
- Popoff V, Mardones GA, Bai SK, Chambon V, Tenza D, Burgos PV, Shi A, Benaroch P, Urbé S, Lamaze C, et al. Analysis of articulation between clathrin and retromer in retrograde sorting on early endosomes. Traffic 2009; 10:1868 - 80; http://dx.doi.org/10.1111/j.1600-0854.2009.00993.x; PMID: 19874558
- Vilariño-Güell C, Rajput A, Milnerwood AJ, Shah B, Szu-Tu C, Trinh J, Yu I, Encarnacion M, Munsie LN, Tapia L, et al. DNAJC13 mutations in Parkinson disease. Hum Mol Genet 2014; 23:1794 - 801; http://dx.doi.org/10.1093/hmg/ddt570; PMID: 24218364
- Neefjes J, van der Kant R. Stuck in traffic: an emerging theme in diseases of the nervous system. Trends Neurosci 2014; 37:66 - 76; http://dx.doi.org/10.1016/j.tins.2013.11.006; PMID: 24411104
- Vilariño-Güell C, Wider C, Ross OA, Dachsel JC, Kachergus JM, Lincoln SJ, Soto-Ortolaza AI, Cobb SA, Wilhoite GJ, Bacon JA, et al. VPS35 mutations in Parkinson disease. Am J Hum Genet 2011; 89:162 - 7; http://dx.doi.org/10.1016/j.ajhg.2011.06.001; PMID: 21763482
- Zimprich A, Benet-Pagès A, Struhal W, Graf E, Eck SH, Offman MN, Haubenberger D, Spielberger S, Schulte EC, Lichtner P, et al. A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am J Hum Genet 2011; 89:168 - 75; http://dx.doi.org/10.1016/j.ajhg.2011.06.008; PMID: 21763483
- Zavodszky E, Seaman MNJ, Moreau K, Jimenez-Sanchez M, Breusegem SY, Harbour ME, Rubinsztein DC. Mutation in VPS35 associated with Parkinson disease impairs WASH complex association and inhibits autophagy. Nat Commun 2014; 5:3828; http://dx.doi.org/10.1038/ncomms4828; PMID: 24819384
- Valdmanis PN, Meijer IA, Reynolds A, Lei A, MacLeod P, Schlesinger D, Zatz M, Reid E, Dion PA, Drapeau P, et al. Mutations in the KIAA0196 gene at the SPG8 locus cause hereditary spastic paraplegia. Am J Hum Genet 2007; 80:152 - 61; http://dx.doi.org/10.1086/510782; PMID: 17160902
- Viklund IM, Kuznetsov NV, Löfberg R, Daperno M, Sostegni R, Astegiano M, Rizzetto M, von Stein O, D’Amato M, von Stein P, et al. Identification of a new WASP and FKBP-like (WAFL) protein in inflammatory bowel disease: a potential marker gene for ulcerative colitis. Int J Colorectal Dis 2008; 23:921 - 30; http://dx.doi.org/10.1007/s00384-008-0527-8; PMID: 18654788
- Rideout HJ, Stefanis L. The neurobiology of LRRK2 and its role in the pathogenesis of Parkinson disease. Neurochem Res 2014; 39:576 - 92; http://dx.doi.org/10.1007/s11064-013-1073-5; PMID: 23729298
- MacLeod DA, Rhinn H, Kuwahara T, Zolin A, Di Paolo G, McCabe BD, Marder KS, Honig LS, Clark LN, Small SA, et al. RAB7L1 interacts with LRRK2 to modify intraneuronal protein sorting and Parkinson disease risk. Neuron 2013; 77:425 - 39; http://dx.doi.org/10.1016/j.neuron.2012.11.033; PMID: 23395371
- Liu Z, Lenardo MJ. The role of LRRK2 in inflammatory bowel disease. Cell Res 2012; 22:1092 - 4; http://dx.doi.org/10.1038/cr.2012.42; PMID: 22430149
