Abstract
Many see fruit flies as an annoyance, invading our homes with a nagging persistence and efficiency. Yet from a scientific perspective, these tiny animals are a wonder of multisensory integration, capable of tracking fragmented odor plumes amidst turbulent winds and constantly varying visual conditions. The peripheral olfactory, mechanosensory, and visual systems of the fruit fly, Drosophila melanogaster, have been studied in great detail;1-4 however, the mechanisms by which fly brains integrate information from multiple sensory modalities to facilitate robust odor tracking remain elusive. Our studies on olfactory orientation by flying flies reveal that these animals do not simply follow their "nose"; rather, fruit flies require mechanosensory and visual input to track odors in flight.5,6 Collectively, these results shed light on the neural circuits involved in odor localization by fruit flies in the wild and illuminate the elegant complexity underlying a behavior to which the annoyed and amazed are familiar.
We used a free-yaw magnetic tether flight simulator surrounded by a circular LED arena and equipped with a spatially discreet odor plumeCitation6,Citation7 to determine the influence of multiple sensory stimuli on olfactory orientation: a hungry fly actively turning toward an attractive apple cider vinegar stimulus. When flies are positioned, for example, 90 degrees to the right of an odor plume they execute a series of left turns, or saccades, directed toward the plumeCitation5 () indicating that these animals are capable of detecting and responding to a spatial odor gradient in flight. This seemingly simple task requires the perception of the odor gradient and a resulting asymmetrical activation of thoracic steering muscles underlying saccades. However, it is currently unclear whether olfactory circuits influence steering behavior independently and parallel to other sensory modalities or if pre-motor neurons controlling olfactory mediated saccades receive multi-modal sensory input.
Olfactory orientation begins when odor molecules bind to olfactory receptors in the dendritic membranes of olfactory receptor neurons (ORNs, , orange) housed in sensilla on the surface of the 3rd antennal segments (a3) and maxillary palps.Citation8,Citation9 We found that eliminating olfactory input to, for example, the left antennal ORNs by occluding the left a3 with glue abolishes leftward olfactory orientationCitation5 (). This suggests that antennal ORNs, rather than maxillary palp ORNs, are critical for detecting the spatial odor gradient. Although some ORNs project unilaterallyCitation9 and could theoretically preserve gradient information, the ORNs thought to underlie behavioral responses to apple cider vinegar project bilaterally to both the left and right antennal lobesCitation10 suggesting that these ORNs might have an as yet uncharacterized capacity to relay gradient information to second order olfactory projection neurons (PNs). PNs innervate higher order olfactory processing centers, namely the mushroom body (MB) and the lateral hornCitation11 (LH, , orange). However anatomical studies on Drosophila have not revealed robust connections between these centers and premotor interneurons that relay information from the brain to the thoracic motor centers.Citation12,Citation13 This suggests that olfactory information follows an indirect route to influence flight behavior, a route possibly mapped by the peculiar requirement of the wind sensing mechanosensory system for olfactory orientation in flight.
Flying Drosophila readily fly upwind,Citation14 a behavior mediated through hundreds of stretch sensitive neurons housed in the second antennal segment (a2) known collectively as the Johnston’s organ (JO).Citation15,Citation16 JO neurons encode the rotation of a3 relative to a2 induced by external wind, gravity and soundCitation1,Citation3,Citation17 and project to defined stimulus specific regions of the antennal motor and mechanosensory centers (ammc).Citation18 Removing input to the left JO, for example, by immobilizing the left a3 relative to a2, impairs upwind orientation to the left.Citation16 Based on this study and existing neural models for wind sensation we speculate that wind mediated saccades to the left are elicited by the passive clockwise rotation of the a3s by wind () and the resulting activation of zones C and E of the ammc ipsilateral and contralateral to the wind, respectivelyCitation1 (). The requirement of the JO ipsilateral to a wind stimulus suggests that the activation of zone C by JO neurons and possibly through the subsequent activation of pre-motor interneurons arising in the ammcCitation3,Citation19,Citation20 triggers directed saccades ().
Likewise, we found that immobilizing the left JO abolishes leftward olfactory orientation in the absence of wind5 (), suggesting that the olfactory system recruits the wind sensing mechanosensory system to initiate olfactory driven saccades. We propose that the active olfactory recruitment of this system is mediated through a class of neurons found in the Drosophila brain which appear to connect the axon terminals of PNs in the ventral LH to possible regions of interest in the ammcCitation13 (, maroon). We further believe the comparatively higher activation of the left ORNs and PNs, in response to an odor on the left () and through LH-ammc interneurons (), triggers a leftward saccade mediated by the left ammc. However, the requirement of the JO ipsalateral to the odor stimulus suggests that asymmetrical olfactory cues must activate first-order JO neurons to initiate saccades, possibly through asymmetrical activation of motor neurons arising from the ammc and terminating in muscles in the a2sCitation21 (, yellow, and ). We speculate that these muscles serve to actively rotate the a3s to mimic a passive wind stimulus () and trigger an ammc mediated saccade ().
This hypothesis is perhaps not as speculative as it may seem since similar systems exist in other insects.Citation22,Citation23 However, little is known about the actual motor neurons which arise from the Drosophila ammc, the muscles in the a2s, and the actual antennal movements in flight. Coupling of the olfactory and mechanosensory systems seems ethologically advantageous for a fly given that an odor source will generally be located upwind.Citation24 Additionally, upwind flight is enhanced in the presence of an odorCitation14 and both a headwind and attractive olfactory cues trigger increases in wing beat frequency and amplitude.Citation25,Citation26 Most interesting, however, is that the ventral LH is not only a site of olfactory and mechanosensory integration but might receive visual input as well.Citation13
Curiously, flies require visual feedback to localize an odor source in free flightCitation27 and consistent with this study and our previous results,Citation6 we found that flies generally fail to direct saccades toward an attractive odor plume in the absence of panoramic visual feedback (). We suggest that, despite their unknown polarity,Citation11 this dependence is due to a class of neurons in Drosophila that connect the optic glomeruli of the ventrolateral protocerebrum (vlpr) to the ventral LHCitation13 (, green). We posit that these neurons, similar to those in other insects,Citation28,Citation29 gate incoming olfactory information to the ammc and, considering that the rate of saccade initiation increases in the absence of visual cues,Citation6 might also modulate the frequency of ammc mediated saccades. This also raises the intriguing possibility that, similar to bees,Citation28 visual input might directly activate antennal movements in flies. Furthermore, as the most prominent site for the multisensory convergence relevant to olfactory orientation in Drosophila, the LH might also be involved in the olfactory influence on optomotor flight behaviorCitation30 and the cross-modal enhancement of visual and olfactory memory in flies.Citation31 Contrary to the idea that each sensory modality influences behavior independent of one another via separate and parallel descending tracts, our results are converging upon the notion that olfactory orientation, whether in the wild or in the kitchen, relies heavily on tightly integrated multi-modal reflexes.
Figures and Tables
Figure 1 Olfactory orientation in flight requires multisensory input. (A, top) In a free-yaw magnetic tether flight arena,Citation7 flying flies were positioned with an oscillating vertical stripe 90° to the right (blue arrows) of a 20° apple cider vinegar plume (orange triangle). At time = 0, we switched on a high contrast visual panorama and recorded the subsequent turning behavior by the fly (data for A-C adapted fromCitation5). (A, bottom) Five representative flight trajectories, where color is used to distinguish between individuals, reveal flies direct saccades toward the plume. Occluding the left 3rd antennal segment with glue (B, top) abolishes leftward olfactory orientation (B, bottom). Immobilizing the left Johnston's organ (JO) with glue (C, top) abolishes leftward olfactory orientation (C, bottom). When presented with a uniform visual panorama (D, top) at time = 0, instead of a high contrast visual panorama (A, top), flies fail to orient toward the odor plume (D, bottom). (experimental methods for D as in (A) and;Citation5 visual stimulus as inCitation6)
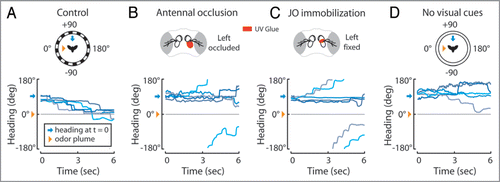
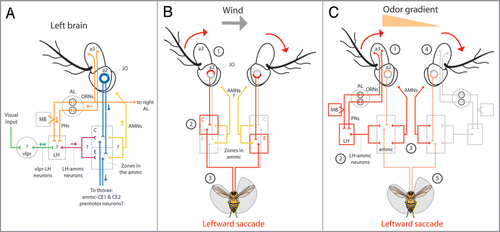
Figure 2 Anatomical model for olfactory orientation. (A) Odor activates first-order olfactory receptor neurons (ORNs) housed in the 3rd antennal segment (a3) which connect unilaterally, or bilaterally to specific second-order olfactory projection neurons (PNs) within defined glomeruli in the antennal lobeCitation9 (AL, orange). PNs connect to either the lateral horn (LH) or the mushroom body (MB) and the (LH).Citation11 The LH presumably receives visual input from the ventrolateral protocerebrum (vlpr) via vlpr-LH interneuronsCitation13 (green) and apparently relays visual and olfactory information to the antennal mechanosensory and motor center (ammc) via LH-ammc interneuronsCitation11,Citation13 (maroon). The ammc receives input from the mechanosensory Johnston’s organ (JO) housed in the second antennal segment (a2, blue). JO neurons project to defined stimulus specific regions of the ammc;Citation18 zones C and E mediate wind sensation.Citation1 Presumably, antennal motor neurons (AMNs, yellow) arise from unknown regions of the ammc and innervate muscles within the a2s to rotate the a3s. Rotation of a3 relative to a2 is encoded by JO neurons which may synapse onto identified pre-motor descending neurons; the dendrites of ammc-CE1 and ammc-CE2 descending neurons innervate zones C and E of the ammc.Citation3 (arrows indicate presumed direction of information flow; (?) indicate unknown connectivity) (B) Wind from the left passively rotates both a3s clockwise (1) and through JO neurons, activates zone C ipsilateral to the wind and zone E contralateral to the wind (2). Gluing the left JO impairs leftward upwind orientationCitation16 suggesting that premotor neurons connecting to zone C of the left ammc activate leftward saccades (3). It is unknown if AMNs participate in this response. (C) As a result of , we believe that the increased activation of the left ORNs and PNs in response to an odor on the left (1, orange triangle) and via LH-ammc neurons (2),Citation13 triggers an asymmetrical activation of AMNs arising from the ammc (3) which, via antennal muscles in the a2s,Citation21 actively rotate the a3s to mimic a passive wind stimulus (4) and trigger a leftward ammc mediated saccade (5, ). (red arrows in B and C indicate rotation of a3 relative to a2; red lines in B and C indicate presumed and exaggerated circuit activation; orange triangle in C indicates higher odor concentration on the left).
Addendum to:
References
- Yorozu S, Wong A, Fischer BJ, Dankert H, Kernan MJ, Kamikouchi A, et al. Distinct sensory representations of wind and near-field sound in the Drosophila brain. Nature 2009; 458:201 - 205
- Stocker RF. The organization of the chemosensory system in Drosophila melanogaster. Cell Tissue Res 1994; 275:3 - 26
- Kamikouchi A, Inagaki HK, Effertz T, Hendrich O, Fiala A, Gopfert MC, et al. The neural basis of Drosophila gravity-sensing and hearing. Nature 2009; 458:165 - 171
- Rister J, Pauls D, Schnell B, Ting CY, Lee CH, Sinakevitch I, Morante J, et al. Dissection of the peripheral motion channel in the visual system of Drosophila melanogaster. Neuron 2007; 56:155 - 170
- Duistermars BJ, Chow DM, Frye MA. Flies require bilateral sensory input to track odor gradients in flight. Curr Biol 2009; 19:1301 - 1307
- Duistermars BJ, Frye MA. Crossmodal visual input for odor tracking during fly flight. Curr Biol 2008; 18:270 - 275
- Duistermars BJ, Frye M. A magnetic tether system to investigate visual and olfactory mediated flight control in Drosophila. J Vis Exp 21::pii 1063 doi: 10.3791/1063
- Vosshall LB, Amrein H, Morozov PS, Rzhetsky A, Axel R. A spatial map of olfactory receptor expression in the Drosophila antenna. Cell 1999; 96:725 - 736
- Stocker RF, Lienhard MC, Borst A, Fischback K-F. Neuronal architecture of the antennal lobe in Drosophila melanogaster. Cell Tissue Res 1990; 262:9 - 34
- Semmelhack JL, Wang JW. Select Drosophila glomeruli mediate innate olfactory attraction and aversion. Nature 2009; 459:218 - 223
- Jefferis GS, Potter CJ, Chan AM, Marin EC, Rohlfing T, Maurer CR, et al. Comprehensive maps of Drosophila higher olfactory centers: spatially segregated fruit and pheromone representation. Cell 2007; 128:1187 - 1203
- Ito K, Suzuki K, Estes P, Ramaswami M, Yamamoto D, Strausfeld NJ. The organization of extrinsic neurons and their implications in the functional roles of the mushroom bodies in Drosophila melanogaster meigen. Learn Mem 1998; 5:52 - 77
- Tanaka NK, Asasaki T, Shimada T, Ito K. Integration of chemosensory pathways in the Drosophila secondorder olfactory centers. Curr Biol 2004; 14:449 - 457
- Budick S, Dickinson M. Free-flight responses of Drosophila melanogaster to attractive odors. J Exp Biol 2006; 209:3001 - 3017
- Eberl DF, Boekhoff-Falk G. Development of Johnston’s organ in Drosophila. Int J Dev Biol 2007; 51:679 - 687
- Budick S, Reiser MB, Dickinson M. The role of visual and mechanosensory cues in structuring forward flight in Drosophila melanogaster. J Exp Biol 2007; 210:4092 - 4103
- Todi SV, Sharma Y, Eberl DF. Anatomical and molecular design of the Drosophila antenna as a flagellar auditory organ. Microsc Res Tech 2004; 63:388 - 399
- Kamikouchi A, Shimada T, Ito K. Comprehensive classification of the auditory sensory projections in the brain of the fruit fly Drosophila melanogaster. J Comp Neurol 2006; 499:317 - 356
- Burdohan JA, Comer CM. Cellular organization of an antennal mechanosensory pathway in the cockroach, Periplaneta americana. J Neurosci 1996; 16:5830 - 5843
- Ye S, Comer CM. Correspondence of escape-turning behavior with activity of descending mechanosensory interneurons in the cockroach, Periplaneta americana. J Neurosci 1996; 16:5844 - 5853
- Hartenstein V. Sink Helen. The muscle pattern of Drosophila 2006; Landes Biosciences / Eurekah.com Springer Science + Business Media ISBN: 0-387-30053-30058.
- Kloppenburg P. Anatomy of the antennal motoneurons in the brain of the honeybee (Apis mellifera). J Comp Neurol 1995; 363:333 - 343
- Homberg V, Montague RA, Hildebrand JG. Anatomy of antenno-cerebral pathways in the brain of the Sphinx Moth, Manduca sexta. Cell Tissue Res 1988; 254:255 - 281
- Vickers NJ. Mechanisms of animal navigation in odor plumes. Biol. Bull 2000; 198:203 - 212
- David CT. Compensation for height in the control of groundspeed by Drosophila in a new ‘barber’s pole’ wind tunnel. J. Comp Physiol 1982; 147:485 - 493
- Frye MA, Dickinson MH. Motor output reflects the linear superposition of visual and olfactory inputs in Drosophila. J Exp Biol 2004; 207:123 - 131
- Frye MA, Tarsitano M, Dickinson MH. Odor localization requires visual feedback during free flight in Drosophila melanogaster. J Exp Biol 2003; 206:843 - 855
- Maronde U. Common projection areas of antennal and visual pathways in the honeybee brain, Apis mellifera. J Comp Neurol 1991; 309:328 - 340
- Strausfeld NJ, Sinakevitch I, Okamura JY. Organization of local interneurons in optic glomeruli of the dipterous visual system and comparisons with the antennal lobes. Dev Neurobiol 2007; 67:1267 - 1288
- Chow DM, Frye MA. Context-dependent olfactory enhancement of optomotor flight control in Drosophila. J Exp Biol 2008; 211:2478 - 2485
- Guo FZ, Guo AK. Crossmodal interactions between olfactory and visual learning in Drosophila. Science 2005; 309:307 - 310