Abstract
Though recent work has demonstrated that plants can recognize species, kin versus strangers, and self/non-self roots, no mechanism for identity recognition in plants has yet been found. Here we examined the role of soluble chemicals in signaling among roots. Utilizing Arabidopsis thaliana, we exposed young seedlings to liquid media containing exudates from siblings, strangers (non-siblings), or only their own exudates. In one experiment, root secretions were inhibited by sodium orthovanadate and root length and number of lateral roots were measured. In a second experiment, responses to siblings, strangers, and their own exudates were measured for several accessions (genotypes), and the traits of length of the longest lateral root and hypocotyl length were also measured. The exposure of plants to the root exudates of strangers induced greater lateral root formation than exposure of plants to sibling exudates. Stranger recognition was abolished upon treatment with the secretion inhibitor. In one experiment, plants exposed to sibling or stranger exudates have shorter roots than plants only exposed to their own exudates. This self/non-self recognition response was not affected by the secretion inhibitor. The results demonstrate that that kin recognition and self/non-self are two separate identity recognition systems involving soluble chemicals. Kin recognition requires active secretion by roots.
Introduction
Identity recognition in plants is involved in multiple biological functions ranging from self-incompatibility pollen-stigma interactions to avoid self fertilization to microbe recognition that allows plants to resist pathogens but form partnerships with mutualistsCitation1 and even to host recognition by parasitic plants that signals haustoria on host roots.Citation2 However, even more subtle forms of recognition and sensing at the plant organ level are being identified.Citation3–Citation5 For example, roots show responses to the neighboring roots’ genotype,Citation6 species,Citation7–Citation8 relatednessCitation9 and whether the neighboring root is self or non-self.Citation6,Citation10–Citation14 While these studies demonstrate that plants are able to alter their root growth according to the presence or absence of specific neighbors, the question remains; how do roots sense and recognize other roots? For animals, context is a common cue of identity,Citation15–Citation17 but these root identity studies control contextual cues, indicating that roots are able to recognize and discriminate identity from the traits of competing roots.
Mahall and CallawayCitation6,Citation13–Citation14 first demonstrated identity recognition by roots. They found self/non-self recognition in the desert shrub Ambrosia dumosa, whose roots stop growing when encountering roots of other plants from the same population but not when encountering roots from the same physiological individual. Subsequent studies found self/non-self recognition with the response in the opposite direction; in strawberry,Citation10 peasCitation12 and buffalo grass,Citation11 root growth is promoted by non-self roots, but not by self roots. Peas show directional growth towards non-self individuals.Citation12 The common feature of self/non-self recognition found by all of these studies is that roots must be physiologically attached to be recognized as self. The roots of detached clones, even though genetically identical, are recognized as non-self.
A parallel suite of studies find kin recognition by roots: groups of siblings demonstrate a different phenotype than groups of strangers, but only when roots are able to interact.Citation9,Citation18 All three studies have found differences in root allocation between groups of kin and groups of strangers sharing a pot. In Cakile edentula, groups of strangers have greater root allocation than groups of siblings.Citation9 Conversely, in two other species, Chenopodium album (Dudley et al., unpublished data) and Impatiens pallida,Citation18 groups of strangers have lower root allocation than groups of siblings.
Kin selection has been argued to be distinct from self/non-self recognition, because in self/non-self recognition genetically identical but physiologically separated clones are recognized as non-self, while in kin recognition, individuals distinguish siblings from strangers, presumably from genetic similarity. Interestingly, some findings may indicate gradations between self and non-self recognition. Self/non-self studies in peas and Buffalo grass,Citation11–Citation12 found that the root traits for interacting detached clones were intermediate between those of attached non-clones and two independent plants, which could indicate a continuum of responses to similarity/difference.
Known plant communication and identity recognition systems involve multiple types of signals: light, cell-cell signaling, and volatiles.Citation19 Gruntman & NovoplanskyCitation11 have suggested that an unknown physiological mechanism, e.g. an electrical or hormonal rhythm, may allow self/non-self recognition. Roots secrete many chemicals into the rhizosphere including secondary compounds such as phenols and flavonoids as well as sugars, organic acids, amino acids and proteins. The cocktail of compounds that are secreted actively or released passively by roots is usually referred to as the root exudate.Citation20 Many of the exudate components are known to behave as allelochemicals between plants and as communication intermediaries in plant-microbe associations between legumes and rhizobia.Citation20 In the noxious weed, Centaurea maculosa, secretion of the allelochemical (±)-catechin into the surrounding rhizosphere mediates intraspecific population regulation. The recognition of (±)-catechin by C. maculosa seeds postpones germination and prevents sibling competition.Citation21 Use of activated carbon, which non-specifically absorbs chemicals, has been shown to block some root sensing phenomena,Citation12–Citation14 suggesting that a soluble chemical is involved in signaling. Although a soluble chemical signal actively secreted from the roots is a potential source for root identity recognition, no one has yet to our knowledge, tried to elicit a root identity response by exposing plants only to soluble components from roots.
Here, we exposed seedlings of Arabidopsis thaliana to liquid media in which roots have grown, rather than to the roots themselves. We also used a recently developed technique to manipulate root secretion. Loyola-Vargas et al.,Citation22 utilizing A. thaliana as a system, have shown that the root exudates profile can be altered by compounds, including potassium cyanide, orthovanadate, quinidine, glibenclamide, nifedipine and verapamil, that are known to inhibit the functioning of various transporters, particularly the ATP-binding cassettes (ABC) transporters. Orthovanadate inhibited secretion by roots of 7 phenolic compounds out of 16 that they identified in the root exudates, but did not affect plant growth.
To examine the response of A. thaliana roots after exposure to root exudates from kin and stranger seedlings, seedlings were placed in liquid media rather than soil. We carried out two studies of identity recognition in A. thaliana, with the first focusing on the effects of a secretion inhibitor, and the second measuring the response across multiple accessions (ecotypes). In the secretion inhibitor study, we compared responses to media in which other seedlings had grown in control conditions to media where seedlings were grown with a root secretion transporter inhibitor (sodium orthovanadate) added. In the multiple accession study, we measured main root length, number of lateral roots, length of the longest lateral root, and hypocotyl length on each plant for several different accessions in control conditions. We ask the following questions: (1) Do A. thaliana seedlings show plasticity to the media in which other seedlings have grown that depends on identity? (2) Does a secretion inhibitor affect that response? (3) Are responses robust to differing accessions, and do they involve multiple traits?
Results
Secretion inhibitor experiment.
In the control treatment, seedlings exposed to media containing STRANGER exudates had more lateral roots than seedlings exposed to KIN or OWN exudates (, ; Supplementary movie 1 & 2). These results clearly indicate that A. thaliana responds differentially to exudates of kin or strangers. Root length for the target sibship showed a different pattern, with the OWN seedlings having longer roots than seedlings exposed to KIN or STRANGER exudates (, ), a pattern expected in self/non-self recognition.
Addition of a pump inhibitor and known root secretion inhibitor sodium orthovanadate (Na3VO4) (3 μM) eliminated the differences in the number of lateral roots produced by the target sibship between the KIN and STRANGERS treatments (, ). The administration of Na3VO4 slightly reduced the primary root length (, ) but did not eliminate the difference between OWN seedlings and KIN or STRANGERS seedlings.
Multiple accession experiment.
The seedlings exposed to STRANGER exudates had more lateral roots than seedlings exposed to KIN or OWN exudates (, ). There was a large difference in number of laterals among trials (), which can be best explained genetic differences in allocation () rather than differences in overall growth, since a measure of seedling size, the length of the main axis (root length + hypocotyl length) did not differ between exudate source treatments or trials (exudate source treatment F2,4 = 0.02 N.S. and trial F2,4 = 4.94, N.S.). All accessions responded similarly to exudates, as shown by a lack of significant exudate source × trial or exudate source × accession nested in trial effects, demonstrating this differential response of A. thaliana to STRANGERS is robust to the genetic background.
Unlike the results of the secretion inhibitor experiment, root length did not differ among the exudate source treatments, and no trait showed a self/non-self response. Number of lateral roots was positively correlated with length of main root and longest lateral, but negatively correlated with hypocotyl length (Table 3). Seedlings exposed to STRANGER exudates tended to have longer lateral roots and shorter hypocotyls, though these differences were not significant.
Discussion
Recent experimental studies of belowground neighbor and identity recognition in plants have generated excitement at the implications of recognition,Citation3–Citation4 but have been criticized for potential pitfalls in experimental setups that could invalidate the conclusions.Citation23–Citation27 The difficulty remains in the complexity of plant effects on the environment: neighbors draw down limited resources, alter the microclimate, and provide cues that elicit developmental plasticity. Identity recognition studies have grown plants together, experimentally controlling resource levels and plant proximity while varying the presence or identity of neighboring roots. These studies are particularly critiqued for flawed control of complicating factors. Plants could be responding to a local depletion in mineral nutrients by the competitor, or to the pot size differences that are used to maintain proportionality of plants to resources, rather than cues of competitors. The present study is therefore unique and important in presenting seedlings with only a potential cue in a highly controlled setup. Here we found that newly germinated seedlings in sterile culture showed identity recognition to unknown root exudates. Two different root traits demonstrated distinct patterns of plasticity to identity that correspond to patterns seen in identity recognition studies in larger plants. Our experimental setup allows us to make some inferences about mechanisms that plants use to sense the identity of other plants.
The most controversial root recognition studies are the presence/absence studies that find increased root allocation in plants in large shared pots compared to plants in smaller solitary pots.Citation28–Citation29 Our present study has echoed these past results in showing changes in root growth with media conditioned by competitors. Factors that potentially confounded the results in pot experiments were eliminated here, since wells within plates are a constant size, and nutrients remained controlled. The experimental design permits us to definitively conclude that a soluble chemical conveys identity, rather than any cue communicated by contact between roots. Actual contact between seedlings was prevented by the experimental design. Seed were initially sown in single accessions on solid plates at low density, so that newly germinated seedlings were not touching. Later in the experiments, seedlings were never present in the same liquid media at the same time. Volatile chemicals could potentially disseminate within a petri dish and within a multi-well plate through the shared headspace. But, though exchange of volatiles between plants occurred, we conclude that any accession-specific volatiles were neither sufficient nor required for the kin/stranger identity response to occur, because the same difference between STRANGER versus KIN and OWN was found in both experiments: in the inhibitor experiment each well plate contained a single accession; and in the multiple accession experiments all accessions were present in the same well plate for every treatment. Thus, under our experimental conditions, the only cue required for stranger recognition was contact with conditioned media from other individuals, and potential contact with volatiles from other accessions alone did not elicit a response. The experimental design cannot rule out a non-specific volatile as a secondary cue, and preliminary data (results not shown) suggest that the stranger response is greatest in plates containing only seedlings exposed to stranger media.
We next consider the most likely source of the specific soluble cue. Seedlings were maintained in sterile conditions, eliminating any role of plant-microbe or microbe-microbe interactions. The seedlings were entirely immersed in the liquid media, so it is possible that leachates from aboveground parts could be a source of identity cues. The most likely source of the signal, however, is exudation from roots, because roots secrete compounds actively,Citation20 and the most controlled studies of identity recognition,Citation13–Citation14,Citation22 localize the site of recognition to roots. Many of these root exudate components behave as communication intermediaries in allelopathic interactions among plants and in plant-microbe associations between legumes and rhizobia.Citation20 To further support our theory that root exudates are responsible for identity recognition, we tested the effects of the root secretion inhibitor sodium orthovanadate (Na3VO4), which has been shown to block active root secretion of several phenolic compounds in A. thaliana.Citation22 Our results clearly illustrate that sodium orthovanadate prevented the seedlings from recognizing strangers, though it did not affect the difference in root length between own and other treatments indicating that the inhibitor was not harmful at the concentration used in the experiments. Whether the signal was one previously identified as blocked by orthovanadate is unknown, since Loyola-Vargas et al. measured a subset of the large number of compounds that roots secrete.Citation22
Kin recognition studies find that root allocation in groups of siblings is similar to that of plants in solitary pots, while groups of strangers either increase root allocation, in Cakile edentula,Citation9 or decrease root allocation, in Chenopodium album (Dudley et al., unpublished results) and Impatiens capensis.Citation20 In the present experiment, the number of lateral roots demonstrated kin/stranger recognition. The number of lateral roots was greatest in seedlings exposed to stranger exudates, and plants exposed only to their own exudates or the exudates of siblings had fewer lateral roots. Since the two experiments were carried out with mutual consultation and use of some of the same seed families, but were done independently, the cross-comparison indicates the robustness of the response. Increased lateral root production with strangers was found in both sets of experiments done with slightly different methodologies, and whether the strangers were from the same population, or from geographically distant populations. The sensitivity of the response to sodium orthovanadate, a secretion inhibitor, localizes the source and nature of the cue of kin recognition to a chemical cue exuded by the root. Though accessions differed in the number of lateral roots, there was no difference among accessions in how lateral root number responded to strangers. A tradeoff between production of lateral roots and hypocotyl elongation suggests that increasing belowground competitiveness decreases potential aboveground competitiveness.
The evidence of self/non-self communication between plant roots has been demonstrated for several plant species including Pisum sativum, Ambrosia dumosa, Larrea tridentate, Buchloe dactyloides and Fragaria chileonsis.Citation10–Citation12,Citation14 Differential responses to physiologically collected roots versus any other roots regardless of their genetic similarity characterize self/non-self recognition. In the present study, root length demonstrated apparent self/non-self recognition. Root length was longest in seedlings only exposed to their own exudates, and shorter in seedlings exposed to the exudates of siblings or strangers, and the response was not affected by the vanadate inhibitor. However, this response was only found in one sodium orthovandate experiment, suggesting that either the root length response was sensitive to technique or that it varied among accessions. It was only found in the secretion inhibitor experiment and in some but not all other accessions in preliminary experiments by M Biedrzycki (unpublished data). It may be that none of the other accessions in the multiple accession experiment had that response. But it is also possible that small differences in sowing density and moisture during the initial growth period on solid media resulted in differences in how much exposure to kin occurred before seedlings were isolated in wells.
The finding of a potential soluble chemical cue for self/nonself seems surprising, given that physiological connectedness is common to all studies showing self/non-self recognition. Mahall & CallawayCitation14 show that activated charcoal, which absorbs many compounds, prevents general root inhibition in Larrea tridentata, but does not interfere with self/non-self recognition in Ambrosia dumosa. Root navigation around physical obstacles is prevented by activated charcoal or potassium permanganate, which oxidizes organic compounds.Citation30 It is difficult to imagine that individual plants produce and recognize a unique chemical fingerprint. More plausible are hypotheses from Gruntman & NovaplanskyCitation11 and Falik et al.Citation30 that plants could produce oscillating levels of some chemical, and respond to levels or gradients of those chemicals. Brief removal and replacement of seedlings would not disrupt any potential feedback between the seedling and its media.
Plants depend on their dynamic and industrious root systems to provide nutrients and water to the rest of the plant, often competing with other plants for these necessities. Lateral root growth in plants has been shown to be a very plastic and nutrient-dependent process.Citation31 Here we show that root morphology of very young seedlings of Arabidopsis also responds to identity, supporting the contention that kin selection can be a major force in the evolution of plants. Our results provide another report of kin recognition and self/non-self recognition. Though it has been argued that kin recognition is a manifestation of self/non-self recognition,Citation31 by finding one response consistent with kin/stranger and another consistent with self/non-self in the same study, we demonstrate that these plant identification patterns and sensory systems are distinct. Most importantly, we were able to localize the cue of identity to a soluble cue, establishing root exudates as the mechanism through which plants recognize kinship and revealing a role of root secreted metabolites in social interactions among plants. In the future, having shown that kin discrimination is a root exudate-dependent phenomenon, we will further elucidate the chemical constituents in the root exudates of KIN exposed to STRANGERS plants to examine the secretion flux patterns in plants under these regimes. The question of how plants recognize kin or strangers can be approached through the genetic and molecular tools available in Arabidopsis.
Materials and Methods
(A) Study species and accessions.
These studies used Arabidopsis thaliana accessions from multiple sources. For Arabidopsis, the terms ecotype and accession are used synonymously,Citation32 but accession is the most appropriate term for a collection made at a specific location and time.Citation33 Several natural accessions were collected by Dr. K. Donohue (Harvard University) from a single North American population near the Charles River in Watertown, MA (CHA prefix) and maintained by single seed descent for at least two generations in the lab. The secretion inhibitor experiment used a selfed family of CHA25 as the target accession and Col(0) seeds procured from Lehle seeds (TX) as the stranger accession. In the multiple accession experiment, the first trial included CHA11 and CHA38 accessions. The second trial included Col(0) which had been lab-maintained for over 10 generations and WS(0) sourced from Lehle seeds and then lab-maintained for at least three generations. The latter two accessions were allowed to self, but the seed we used may have been bulked across multiple mothers. The third trial included CHA4, CHA28 CHA31, and CHA34 accessions.
(B) Experimental design.
Two complementary experiments determining the effects of relatedness on seedling phenotype were carried out independently. One incorporated a secretion inhibitor to explore mechanism, but only measured how one accession responded to a second accession. The second experiment measured responses across multiple accessions and included more traits. Differences in methodologies are identified.
Secretion inhibitor experiment.
This experiment was designed to determine root response to media conditioned by individuals of differing relatedness, and if a secretion inhibitor would affect the response. All measured seedlings were of a single target accession, with a second accession used as strangers. The three exudate source treatments were OWN (control), KIN (sibling), and STRANGER (non-sibling). The secretion inhibitor treatments were control and sodium orthovanadate. The experiment was factorial, with a single combination of the exudate source × secretion inhibitor treatments, .e.g., OWN control, applied to an individual well plate of 24 seedlings. Three trials comprising two replicate plates of 24 seedlings each per trial, were carried out. The total sample size (N) was therefore 3 exudate source × 2 secretion inhibitor × 3 trials × 2 plates × 24 seedlings per plate = 864 seedlings. However, the number of seedlings measured for each plate was in some cases less than 24 seedlings, as any damaged seedlings were not recorded. Each plate contained only a single accession. This experiment was carried out by Meredith Biedrzycki at the University of Delaware.
Multiple accession experiment.
This experiment comprised eight accessions. An additional two traits, longest lateral root length and hypocotyl length, were measured. In this experiment all seedlings were measured, with each accession therefore used both as a target and a stranger. The three exudate source treatments were OWN, KIN, and STRANGER. These treatments were applied to one plate of 24 seedlings per trial, and 3 trials were carried out, for a total of N=216 for the study. Limited quantities of seed and germination difficulties prevented the use of all accessions in all trials. This experiment was carried out by Tafari Jilany at McMaster University.
(C) Culture conditions.
Secretion inhibitor experiment.
Seeds were surface sterilized with 50% sodium hypochlorite for 3 minutes then washed twice with sterile ddH2O. CHA seeds required one week storage in a refrigerator for germination. Seeds were sown in low density, approximately 80–100 seeds of a single accession, evenly spaced on Murashige and SkoogCitation34 media (3% sucrose) plates (100x15 mm) for seven days. On the seventh day, under sterile conditions, seedlings were added individually to 3.5 ml wells of a 24 well tissue culture plate (85.5 mm x 127.5 mm, BD Falcon NY, USA) with 1mL of MS liquid media per seedling (1% sucrose) with or without sodium orthovanadate. Plates were placed on a rotary shaker (90 rpm) under cool white fluorescent light (45 mmol m−2 s−1) at 25 ± 2 °C. The rotary shaker was used to maintain mixing in the solution and so prevent hypoxia. Order of plates on the shaker was re-randomized daily. Exudate source and inhibitor treatments were imposed for seven days, and then the plants were measured, as described below.
Multiple accession experiment.
Techniques as above, except that seeds were surface sterilized with 1mL of 70% ethanol for 2 minutes and then washed with a 1mL solution of 30% bleach, 0.1% Tween 20 and sterile water for ten minutes. Seeds were then rinsed with 1mL of sterile water five times. Seeds were transferred to centrifuge tubes with 0.1% phytagar and stored in a refrigerator for one week (CHA) or two days (Col(0) and WS(0)). After the two day storage period, seeds were sown on Murashige and SkoogCitation34 media (1% sucrose, pH 5.8). Growth on solid MS plates was seven days, and exudate source treatments were imposed for five days.
(D) Treatments.
Exposure to exudates from different sources.
Each day for seven days (secretion inhibitor experiment) or four days (multiple accession experiment), target family plants in the “OWN” treatment were lifted out of their wells gently with forceps and placed back into the well containing their exudates. Target family plants in the “KIN” treatment were lifted from their wells gently and placed into a well with media that previously contained a sibling. Target family plants in the “STRANGERS” treatment were lifted out of their wells gently and placed in a well with media that previously contained a stranger plant (Fig. 4). In the STRANGER treatments, each seedling was paired with one other seedling, so that each seedling was only exposed to the exudates of one other seedling, and these two seedlings were switched daily so that they experienced fresh exudates from their partner over the course of the experiment. For the multiple accession experiment, KIN seedlings were also paired, so each was only exposed to the exudates of one other seedling. For the secretion inhibitor experiment, KIN seedlings were moved each day within a well plate, seedlings were shifted one well over, so each experienced the fresh exudates of the one seedling ahead, but were exposed to older exudates from multiple siblings. In both experiments, the OWN seedlings were lifted and replaced daily to control for handling. All seedlings were maintained under sterile conditions.
Control and Sodium orthovanadate treatment.
In order to determine the effect of compromised root exudates on kin recognition, in the secretion inhibitor experiment, the control treatment without added secretion inhibitor, and a treatment of sodium orthovanadate (Na3VO4), an ABC transporter inhibitor, which was added to the media of OWN, KIN and STRANGER treatments.Citation22 All other aspects of the experiment were carried identically, with all combinations sodium orthovanadate and control treatments with the exudate source treatments within each trial. The sodium orthovanadate was added to the growth media in the multi-well 24 well tissue culture plates for the OWN, KIN and STRANGERS treatments before seedlings were added to the wells, for a final concentration in each well to be 3μM Na3VO4.
(E) Measurements.
Secretion inhibitor experiment.
Fourteen days after sowing, seedlings were removed from the media in the wells and the number of lateral roots and length of primary roots on each plant was determined with the unaided eye and recorded. Approximately 1-3 plants per plate were damaged and therefore not recorded.
Multiple accession experiment.
On day nine after sowing, seedlings were removed from the media in the wells, stained with Toluidine blue, and photographed against a white background with a digital camera. The number of lateral roots was counted twice from the images, at least once by an observer blind to treatments. The length of primary roots, longest lateral root, and hypocotyl on each plant was determined from the images by the program Meazure 2.0 (C Thing Software, 2004).
(F) Statistical Analysis.
All statistical analyses were done in PC-SAS (version 8.02; SAS, Cary, NC). Mixed models (both fixed and random effects) were fit in PROC MIXED for continuous traits and in PROC GLIMMIX with a Poisson link function for number of lateral roots. Post-hoc tests were done using the LSMEANs option for PROC MIXED and PROC GLIMMIX.
Secretion inhibitor experiment.
Mixed model analysis of variance was performed, with a plate as a random factor, and trial,Citation35 secretion inhibitor treatment, and exudate source as fixed effects. Using a plate as a random factor limited degrees of freedom for testing effects. All possible interactions were fit in initial models. For number of lateral roots, the three-way interaction between trial, secretion inhibitor treatment, and exudate source gave a low F-ratio and high probability, and so was dropped. Means and standard errors were obtained from the LSMEANS option of PROC MIXED. Means and standard errors from PROC GLIMMIX were presented back-transformed from the natural logarithms for better explanatory value.
Multiple accession experiment.
Mixed model analyses of variance were fit with number of lateral roots, primary root length, longest lateral root length, and hypocotyl length as dependent variables. Exudate source and trial were considered fixed effects, and exudate source×trial, accession nested in trial, and exudate source×accession nested in trial as random effects. N=216. For number of lateral roots, a generalized linear model was fit with a Poisson link function.
Figures and Tables
Figure 1 Lateral roots in Arabidopsis thaliana plants grown solitary (OWN) or in sibling (KIN) and non-sibling (STRANGER) exudates from the secretion inhibitor experiment. (A) Shows the representative photographs of OWN, KIN and STRANGER seedlings in the control treatment. (B) Shows the representative photographs of OWN, KIN and STRANGER treatments in the sodium vanadate treatment. (C) Shows the average number of lateral roots among OWN, KIN and ortho STRANGER seedlings in control and sodium vanadate (Na3VO4 [3 µM]) treatments. Means with the same letter do not differ significantly. Ortho bars indicate mean± S.E. for N = 864.
![Figure 1 Lateral roots in Arabidopsis thaliana plants grown solitary (OWN) or in sibling (KIN) and non-sibling (STRANGER) exudates from the secretion inhibitor experiment. (A) Shows the representative photographs of OWN, KIN and STRANGER seedlings in the control treatment. (B) Shows the representative photographs of OWN, KIN and STRANGER treatments in the sodium vanadate treatment. (C) Shows the average number of lateral roots among OWN, KIN and ortho STRANGER seedlings in control and sodium vanadate (Na3VO4 [3 µM]) treatments. Means with the same letter do not differ significantly. Ortho bars indicate mean± S.E. for N = 864.](/cms/asset/8b651226-b2ce-4d43-a31a-07ef487cf718/kcib_a_10910118_f0001.gif)
Figure 2 Average length of primary roots in Arabidopsis thaliana plants grown solitary (OWN) or in sibling (KIN) and non-sibling (STRANGER) exudates from the secretion inhibitor experiment, for the control and sodium orthovanadate [Na3VO4 (3 µM)] treatments. Means with the same letter do not differ significantly. Bars indicate mean± S.E for N = 864.
![Figure 2 Average length of primary roots in Arabidopsis thaliana plants grown solitary (OWN) or in sibling (KIN) and non-sibling (STRANGER) exudates from the secretion inhibitor experiment, for the control and sodium orthovanadate [Na3VO4 (3 µM)] treatments. Means with the same letter do not differ significantly. Bars indicate mean± S.E for N = 864.](/cms/asset/246286bb-e9dd-40d1-9e5a-b87d3f21e00e/kcib_a_10910118_f0002.gif)
Figure 3 Average lateral root number for eight accessions of A. thaliana in OWN (solitary), KIN (from siblings), and STRANGER (non-siblings) exudates. Trial 1 comprises CHA 11, CHA 38, trial 2 Col(0) and WS(0), and trial 3 CHA 28, CHA 31, CHA 34, and CHA 4. Average lateral root number is greater in Col(0) and WS (0) accessions than CHA accessions, but the effects of exudates did not differ among accession. Bars indicate means ± 1S.E for N = 216.
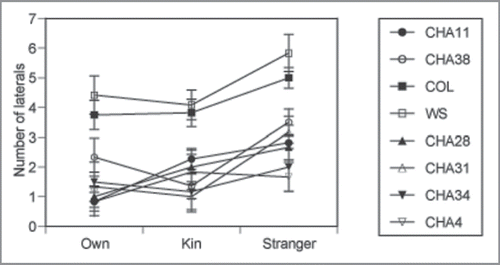
Figure 4 The panel A, B, C represents the experimental set up for OWN, KIN and STRANGER treatments. The arrows in the panel indicates the transfer of plants into exposure to KIN/STRANGER root exudates.
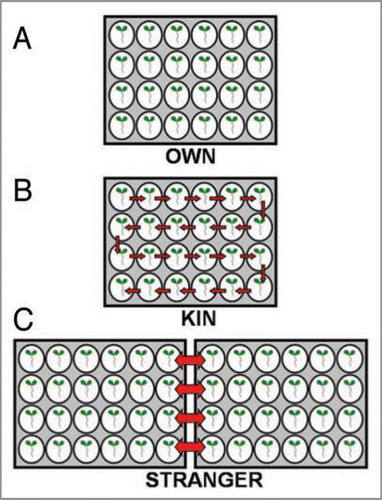
Table 1 Mixed model analysis of variance for the secretion inhibitor experiment. Fixed effects are exudate source (OWN, KIN and STRANGER), secretion inhibitor (control and sodium orthovanadate) and trial. Well plates are considered random effects nested within the three way interaction of exudate source, secretion inhibitor, and trial. Degrees of freedom for numerator and denominator, and F-ratios are reported for fixed effects, and z-values for random effects. For lateral roots, initial analysis showed that the three way interaction between kin, exudate inhibitor, and trial was non-significant with F4.18=0.29 and P> 0.8833. Because the limited degrees of freedom severely compromise the power of the analysis, we dropped that non-significant three-way interaction.
Table 2 Mixed model analysis of variance for the multiple accession experiments. Fixed effects are exudate source (OWN, KIN and STRANGER) and trial. Only one well plate was done for each treatment at each trial, so the trial × exudate source interaction is considered a random effect. Each accession was included in only one trial, so accession is nested within trial as a random effect, and the interaction of exudate source × genotype nested with trial is also a random effect. Degrees of freedom for numerator and denominator, and F-ratios are reported for fixed effects, and z-values for random effects.
Table 3 Pearson correlations among seedling traits from the multiple accession experiment.
Additional material
Download Zip (2.6 MB)Acknowledgments
H.P.B acknowledges the University of Delaware-EPSCoR for faculty start-up grant and NSF (0713774). S.A.D. acknowledges the Natural Science and Engineering Research Council of Canada for research funding. Authors also thank Drs. Thomas E. Hanson, Madhavi Avadhani and Nicole Donofrio for critical reading of the manuscript and editing suggestions, Kathleen Donohue and Robin Cameron for providing seed, and Sinah Lee and Delicia S. Moonilal for technical help. S.A.D. thanks Robin Cameron for her generous help with techniques and supplies.
References
- Sanabria N, Goring D, Nürnberger T, Dubery I. Self/nonself perception and recognition mechanisms in plants: a comparison of self-incompatibility and innate immunity. New Phytol 2008; 178:503 - 514
- Keyes WJ, Palmer AG, Erbil WK, Taylor JV, Apkarian RP, Weeks ER, et al. Semagenesis and the parasitic angiosperm Striga asiatica. Plant J 2007; 51:707 - 716
- Callaway RM. The detection of neighbors by plants. Trends in Ecol Evol 2002; 17:104 - 105
- de Kroon H. Ecology — How do roots interact?. Science 2007; 318:1562 - 1563
- Callaway RM, Mahall BE. Plant ecology: Family roots. Nature 2007; 448:145 - 147
- Mahall BE, Callaway R.M. Effects of regional origin and genotype in intraspecific root communication in the desert shrub Ambrosia dumosa (Asteraceae). Am J of Botany 1996; 83:93 - 98
- Huber-Sannwald E, Pyke DA, Caldwell MM. Morphological plasticity following species-specific recognition and competition in two perennial grasses. Am J of Botany 1996; 83:919 - 931
- Semchenko M, John EA, Hutchings MJ. Effects of physical connection and genetic identity of neighbouring ramets on root-placement patterns in two clonal species. New Phytol 2007; 176:644 - 654
- Dudley SA, File AL. Kin recognition in an annual plant. Biol Letters 2007; 3:435 - 438
- Holzapfel C, Alpert P. Root cooperation in a clonal plant: connected strawberries segregate roots. Oecologia 2003; 134:72 - 77
- Gruntman M, Novoplansky A. Physiologically mediated self/non-self discrimination in roots. PNAS 2004; 101:3863 - 3867
- Falik O, Reides P, Gersani M, Novoplansky A. Self/non-self discrimination in roots. J of Ecol 2003; 91:525 - 531
- Mahall BE, Callaway RM. Root communication among desert shrubs. PNAS 1991; 88:874 - 896
- Mahall BE, Callaway RM. Root communication mechanisms and intra-community distributions of two Mojave Desert shrubs. Ecol 1992; 73:2145 - 2151
- Schausberger P. Kin recognition by juvenile predatory mites: prior association or phenotype matching?. Behav Ecol and Sociobiol 2007; 62:119 - 125
- Waldman B. The ecology of kin recognition. Ann Rev Ecol Stat 1988; 19:543 - 571
- Hauber ME, Safran JE. Behavioural ecology: Promiscuous fathers sire young that recognize true family. Curr Biol 2006; 16:R797 - R800
- Murphy GP, Dudley SA. Kin recognition: competition and cooperation in Impatiens (Balsaminaceae). Am J of Botany 2009; 96:1990 - 1996
- Karban R. Plant behaviour and communication. Ecol Letters 2008; 11:727 - 739
- Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM. The role of root exudates in rhizosphere interactions with plants and other organisms. Ann Reviews in Plant Biol 2006; 57:233 - 266
- Perry LG, Thelen GC, Weir TL, Callaway RM, Paschke MW, Vivanco JM. Dual role for an allelochemical: (±)-catechin from Centaurea maculosa root exudates regulates conspecific seedling establishment. J of Ecol 2005; 93:1126 - 1135
- Loyola-Vargas VM, Broeckling CD, Badri D, Vivanco JM. Effect of transporters on the secretion of phytochemicals by the roots of Arabidopsis thaliana. Planta 2007; 225:301 - 310
- Schenk HJ. Root competition: beyond resource depletion. J of Ecol 2006; 94:735 - 739
- Hess L, de Kroon H. Effects of rooting volume and nutrient availability as an alternative explanation for root self/non-self discrimination. J of Ecol 2007; 95:241 - 251
- Klemens J. Kin recognition in plants?. Biol Letters 2008; 4:67 - 68
- O’Brien EE, Brown SS. Games roots play: effects of soil volume and nutrients. J of Ecol 2008; 96:438 - 446
- Semchenko M, Hutchings MJ, John EA. Challenging the tragedy of the commons in root competition: confounding effects of neighbor presence and substrate volume. J of Ecol 2007; 95:252 - 260
- Gersani M, Brown JS, O’Brien E, Maina G, Abramsky Z. Tragedy of the commons as a result of root competition. J of Ecol 2001; 89:660 - 669
- Murphy GP, Dudley SA. Above- and belowground competition cues elicit independent responses. J of Ecol 2007; 95:261 - 272
- Falik O, Reides P, Gersani M, Novoplansky A. Root navigation by self inhibition. Plant Cell and Environ 2005; 28:562 - 569
- Malamy JE, Ryan KS. Environmental regulation of lateral root initiation in Arabidopsis. Plant Physiol 2001; 127:899 - 909
- Mitchell-Olds T. Arabidopsis thaliana and its wild relatives: a model system for ecology and evolution. Trends in Ecol Evol 2001; 16:693 - 700
- Page DR, Grossniklaus U. The art and design of genetic screens: Arabidopsis thaliana. Nature Reviews Genetics 2002; 3:124 - 136
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia plantarum 1962; 15:473 - 497
- Newman JA, Bergelson J, Grafen A. Blocking factors and hypothesis tests in ecology: is your statistics text wrong?. Ecology 1997; 78:1312 - 1320