Abstract
Myosin-1a is one of eight monomeric, membrane binding class I myosins expressed in vertebrates.1 As the most abundant actin-based motor protein found in the enterocyte microvillus, myosin-1a has long been known to interact with the apical membrane via a highly basic C-terminal tail domain.2 Several recent studies shed light on possible functional consequences of this protein/lipid interaction. In vitro and in vivo studies of microvillar function have revealed that myosin-1a can move apical membrane along core actin bundles, leading to the release of small vesicles from microvillar tips.3,4 Additional studies indicate that myosin-1a and other class I myosins contribute to membrane-cytoskeleton adhesion, which enables the apical membrane to resist deformation.5 These findings clearly position myosin-1a as an important player in apical membrane movement and structural stability. How this motor is able to fulfill these two seemingly distinct functions is currently unclear, but will serve as the focus of our discussion below.
The intestinal epithelial cell brush border is one of the most highly organized F-actin arrays observed in biology. Brush borders consist of thousands of slender, finger-like protrusions of apical membrane known as microvilli, which are supported by parallel bundles of F-actin. Microvilli extend from the cell surface to an identical length and exhibit near perfect hexagonal packing.Citation6 Not surprisingly, this actin-rich assemblage is composed of a wide variety of actin-binding proteins, many of which function in controlling the dynamics of F-actin assembly/disassembly; they also serve to modulate the mechanical properties and dimensions of the supporting actin bundle.Citation7 In addition, this domain is home to a variety of actin-based motor proteins known as “myosins”. While our understanding of myosin function in this biological setting has been developing for many years, the latest studies have highlighted some unexpected and unconventional applications for myosin mechanical activity within the microvillus. These investigations have focused on the class I monomeric membrane-binding motor known as myosin-1a (Myo1a); an abundant microvillar component that links the overlying plasma membrane to the underlying actin bundle. Recent cell biological and biophysical studies have highlighted novel roles for this motor in the unique cytoskeletal context provided by the brush border.
Two recent investigations suggest that microvillar Myo1a is mechanically active and can power the plus-end directed (tipward) sliding of apical membrane along core actin bundles.Citation3,Citation4 In the context of intact, native enterocytes, this activity may be linked to the production and shedding of small vesicles from the tips of microvilli. Biochemical analysis of these vesicles has revealed that one of the most prominent protein cargoes is intestinal alkaline phosphatase, a brush border enzyme that was recently recognized as a gut host defense factor.Citation8,Citation9 The precise mechanism underlying microvillar membrane shedding has not been elucidated, but Myo1a is most likely generating plus-end directed forces that lead to the tip-ward movement of specific vesicle components and accumulation of apical membrane at microvillar tips.
Other recent experiments have focused on a seemingly distinct aspect of myosin- I function in the microvillus: the control of apical membrane tension.Citation5 Pioneering studies on cell membrane mechanics established that physical bonding with the actin cytoskeleton accounts for the majority of tension (i.e., in plane stiffness) observed in cellular plasma membranes.Citation10 For example, regions of the cell membrane that lose contact with the underlying actin cytoskeleton are prone to form spherical membrane protrusions known as “blebs”.Citation11 In the complex case provided by the brush border, the amount of apical membrane supported by protruding microvillar actin bundles is ∼100-fold greater than a simple flat surface would allow. Stabilizing this massive excess of membrane requires high levels of membrane-cytoskeleton (M-C) adhesion energy. Because Myo1a has the potential to serve as a bivalent linkage, simultaneously binding to the inner leaflet of the apical membrane and the core actin bundle, this motor is well suited to contribute to M-C adhesion. Indeed, bleb-like herniations were observed on the apical surface of enterocytes from Myo1a KO mouse.Citation12 Direct physical measurements supporting this hypothesis were provided in recent studies that employed optical trapping to directly measure membrane tension in brush borders isolated from Myo1a KO mice or in cultured epithelial cells where the complement of Myo1a was disrupted.Citation5 The results clearly show that Myo1a and other class I myosins play an important role in enabling the membrane to closely follow the contours of the underlying actin cytoskeleton and resist deformation.
Together, these recent investigations suggest that Myo1a has the potential to function in two distinct processes: (1) the movement of apical membrane along the core actin bundle, and (2) the adhesion of apical membrane to the core actin bundle. These findings leave us with a physical conundrum, as directed movement would require Myo1a motors to frequently detach from the actin core during their ATPase cycle. Obviously, detached molecules would be unable to contribute to M-C adhesion, and thus membrane tension. How does the ensemble of Myo1a within the microvillus perform these two seemingly disparate functions? We briefly consider a few possibilities below:
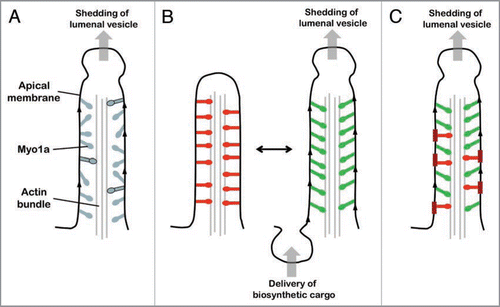
Single population of asynchronously cycling/functioning Myo1a molecules: In this scenario, all Myo1a molecules are functionally equivalent and contribute to both tip-ward movement of apical membrane and M-C adhesion while they are strongly bound to the actin core. Asynchronous ATPase cycling ensures that at least a small ensemble of Myo1a will always be engaged with the actin core bundle (). Here, the duty ratio (i.e., actin-attached duration/total ATPase cycle duration) determines the fraction of the total Myo1a population bound to actin at any instant. Transient kinetic studies suggest that the duty ratio of Myo1a is low, <0.1.Citation17 Because a single microvillus is home to ∼500 Myo1a molecules, at least ∼50 would be strongly bound to the actin core at any moment in time. However, recent single molecule studies indicate that this number has the potential to be much higher. Optical trapping experiments show that even small opposing loads can significantly extend the actin-attached duration and elevate the duty ratio of Myo1b.Citation13 Based on structural and biochemical similarities, Myo1a is expected to demonstrate similar load-dependent kinetics.Citation18 Thus, if attachment to the inner leaflet of the apical membrane provides even a modest level of mechanical resistance (a force of only ∼2 pN is required to increase the actin-attached duration of Myo1b 75-fold),Citation13 the Myo1a duty ratio and number of molecules instantaneously bound to the core actin bundle would increase dramatically. Although a higher duty ratio would slow the velocity of any directed movement powered by Myo1a, it would still enable tip-ward progress of apical membrane while clearly benefiting M-C adhesion.
Single population of Myo1a molecules switching between two functional states: The second scenario again requires that Myo1a molecules are functionally equivalent, but in this case their activity is synchronized. Here, the two functions are performed in a mutually exclusive manner; that is, all Myo1a motors contribute to either M-C adhesion or the tip-ward motility of apical membrane. Switching between these two functional states could be triggered by a chemical or physical signal. One possibility that incorporates the load-dependent kinetics discussed aboveCitation13 is physical switching coupled to the biosynthetic delivery of membrane to the base of microvilli. To illustrate this possibility, we start with the entire Myo1a population contributing only to M-C adhesion (). Mechanical resistance provided by interactions with the membrane would increase the Myo1a duty ratio and facilitate M-C adhesion. However, upon delivery of biosynthetic cargo to the base of the microvillus (e.g., a vesicle from the Golgi bearing apical membrane components), any loading provided by membrane tension would be relieved, allowing Myo1a to continue cycling and enabling the tip-ward movement of apical membrane. This is consistent with previous biophysical studies showing that membrane trafficking and membrane tension are intimately linked, e.g., secretion in the absence of endocytosis would lower plasma membrane stiffness.Citation14 Following one such event, the mechanical resistance provided by the membrane would eventually increase, raising the duty ratio of Myo1a once again. In this manner, the entire population of Myo1a would continue to oscillate between these two functional states.
Coexistence of two functionally distinct Myo1a populations: The third case suggest that the microvillus is home to two distinct populations of Myo1a molecules, one dedicated to M-C adhesion with the other playing a role in moving apical membrane along the core actin bundle (). This idea is supported by evidence from FRAP studies, which revealed that most Myo1a molecules in the microvillus are mobile (∼80%) whereas a small fraction (∼20%) are immobile.Citation15 Immobile Myo1a molecules could be those engaged in M-C adhesion, whereas mobile molecules may be transiently interacting with the actin core bundle, contributing to tip-ward movement of apical membrane. However, these two populations of Myo1a would need to interact with different lipids/ components in the membrane inner leaflet. In this way, immobile Myo1a molecules would not impede the progress of molecules that were attempting to move membrane components toward the tip compartment. Interestingly, biochemical fractionation of the microvillus has shown that ∼20% of the total Myo1a population is associated with detergent insoluble membrane patches.Citation16 Thus, apical membrane micro-domains may provide a physical platform for controlling the dynamics and functional segregation of Myo1a motors in the microvillus.
The three possibilities discussed here represent a subset of models that could explain how Myo1a might carry out multiple distinct functions in a common subcellular structure. Studies exploiting high resolution live cell imaging coupled with new probes that report on the conformation and dynamics of this motor are required before we achieve a satisfactory understanding of the mechanism underlying Myo1a multifunctionality.
Figures and Tables
Figure 1 Models of Myo1a multifunctionality. (A) Single population of asynchronously cycling/functioning Myo1a molecules. In this model, all myosin molecules are functionally equivalent, participating in both the tip-ward movement of apical membrane and M-C adhesion while strongly bound to actin (molecules with black outline). Asynchronous AT Pase cycling means that a fraction of the total Myo1a population will always be strongly bound to the actin core bundle. The strongly bound fraction is controlled by the Myo1a duty ratio, which has the potential to increase dramatically if interactions with the apical membrane present even a minor opposing load. (B) Single Myo1a population switching between two functional states. Red Myo1a molecules are those contributing to M-C adhesion, green molecules are driving tip-ward movement of apical membrane. Delivery of biosynthetic vesicles to the inter-microvillar region transiently reduces tension in the apical membrane and impedance to Myo1a cycling; this gives rise to membrane movement, which leads to the formation of a vesicle at the microvillus tip. (C) Coexistence of two functionally distinct Myo1a populations. The thicker red membrane regions represent detergent insoluble patches that may immobilize a subset of Myo1a molecules (red), allowing them to contribute to M-C adhesion. Myo1a not bound to these patches would be more dynamic (green molecules) and contribute to the tip-ward flow of apical membrane components.
Acknowledgements
This work was supported by grants from the National Institutes of Health (R01 DK-075555, M.J.T.) and the American Heart Association (09GRNT2310188, M.J.T.; Post-doctoral Fellowship 0825358E, R.N.).
Addendum to:
References
- Berg JS, Powell BC, Cheney RE. A millennial myosin census. Mol Biol Cell 2001; 12:780 - 794
- Hayden SM, Wolenski JS, Mooseker MS. Binding of brush border myosin I to phospholipid vesicles. J Cell Biol 1990; 111:443 - 451
- McConnell RE, Higginbotham JN, Shifrin DA Jr, Tabb DL, Coffey RJ, Tyska MJ. The enterocyte microvillus is a vesicle-generating organelle. J Cell Biol 2009; 185:1285 - 1298
- McConnell RE, Tyska MJ. Myosin-1a powers the sliding of apical membrane along microvillar actin bundles. J Cell Biol 2007; 177:671 - 681
- Nambiar R, McConnell RE, Tyska MJ. Control of cell membrane tension by myosin-I. Proc Natl Acad Sci USA 2009; 106:11972 - 11977
- Mooseker MS. Organization, chemistry and assembly of the cytoskeletal apparatus of the intestinal brush border. Ann Rev Cell Biol 1985; 1:209 - 241
- Revenu C, Athman R, Robine S, Louvard D. The co-workers of actin filaments: from cell structures to signals. Nat Rev Mol Cell Biol 2004; 5:635 - 646
- Bates JM, Akerlund J, Mittge E, Guillemin K. Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host Microbe 2007; 2:371 - 382
- Goldberg RF, Austen WG Jr, Zhang X, Munene G, Mostafa G, Biswas S, et al. Intestinal alkaline phosphatase is a gut mucosal defense factor maintained by enteral nutrition. Proc Natl Acad Sci USA 2008; 105:3551 - 3556
- Sheetz MP. Cell control by membrane-cytoskeleton adhesion. Nat Rev Mol Cell Biol 2001; 2:392 - 396
- Charras GT, Hu CK, Coughlin M, Mitchison TJ. Reassembly of contractile actin cortex in cell blebs. J Cell Biol 2006; 175:477 - 490
- Tyska MJ, Mackey AT, Huang JD, Copeland NG, Jenkins NA, Mooseker MS. Myosin-1a is critical for normal brush border structure and composition. Mol Biol Cell 2005; 16:2443 - 2457
- Laakso JM, Lewis JH, Shuman H, Ostap EM. Myosin I can act as a molecular force sensor. Science 2008; 321:133 - 136
- Dai J, Ting-Beall HP, Sheetz MP. The secretioncoupled endocytosis correlates with membrane tension changes in RBL 2H3 Cells. J Gen Phys 1997; 110:1 - 10
- Tyska MJ, Mooseker MS. MYO1A (brush border myosin I) dynamics in the brush border of LLC-PK1- CL4 cells. Biophys J 2002; 82:1869 - 1883
- Tyska MJ, Mooseker MS. A role for myosin-1A in the localization of a brush border disaccharidase. J Cell Biol 2004; 165:395 - 405
- Jontes JD, Milligan RA, Pollard TD, Ostap EM. Kinetic characterization of brush border myosin-I ATPase. Proc Natl Acad Sci USA 1997; 94:14332 - 14337
- De La Cruz EM, Ostap EM. Relating biochemistry and function in the myosin superfamily. Curr Opin Cell Biol 2004; 16:61 - 67