Abstract
The ability of an organism to detect a predator and then to take the appropriate vigilance actions is paramount for survival of the species. Lab-reared snails (> 250 generations) maintain their ability to detect predators and alter both aerial and cutaneous respiration. However, only the scent of a sympatric predator altered aerial respiration in freshly collected ‘wild’ snails. Here we test the hypothesis that the detection of a sympatric predator but not an allopatric predator will alter cutaneous respiration in freshly collected ‘wild’ snails. We find that Alberta snails while altering their cutaneous respiration to the scent of a sympatric predator (Tiger salamander) do not alter respiration to the scent of a crayfish (an allopatric predator). In Dutch snails there is a greater alteration to the scent of crayfish (sympatric predator) than to an allopatric predator (Tiger salamander).
Introduction
We have recently demonstrated that different geographically separate strains (i.e., local, distinct isolated populations) of Lymnaea respond only to the scent of sympatric (i.e., occurring in the same geographic region) predators with alterations in aerial respiratory behaviour, concomitant changes in the activity of RPeD1 (a necessary neuron for that behavior), and an enhanced ability to form long-term memory (LTM) following operant conditioning of aerial respiratory behavior.Citation1 Thus, aerial respiratory was significantly increased in freshly collected Dutch snails or their lab-reared descendants with exposure to crayfish effluent (CE); but CE did not alter aerial respiratory behavior in either freshly collected or lab-reared F1 Alberta snails. Crayfish are not a sympatric predator of these Lymnaea strains. On the other hand aerial respiratory behavior in Dutch snails was not significantly altered by their exposure to Tiger salamander (Ambystoma tigrinum) effluent (SE) whereas exposure to SE caused a significant decrease in aerial respiration in Alberta strains.
Aerial respiration is a simple, easily observable, tractable behavior that is driven by a 3-neuron central pattern generator (CPG) whose sufficiency and necessity has been experimentally determined. However, snails under eumoxic conditions primarily satisfy their respiratory requirements not through aerial respiration but rather via cutaneous respiration.Citation2 Orr et al.Citation3 previously demonstrated that in lab-reared snails (derived from snails collected in The Netherlands), exposure to the scent of the predator altered cutaneous respiration. Thus, we were interested to determine if in wild (i.e., freshly collected) Lymnaea from geographically separate populations (i.e., Alberta and The Netherlands) cutaneous respiration was also altered by the scent of a predator, and if so was it only sympatric predators that altered this form of respiration. We would therefore determine if exposure to only a sympatric predator effluent induced metabolic changes in geographically separate strains of Lymnaea.
Results
O2 consumption.
We sought to determine if the 3 different populations of snails altered their cutaneous respiration in response to exposure to CE and SE as did their aerial respiratory behaviors.Citation1 Therefore non-aerial oxygen consumption (i.e., cutaneous respiration) was measured in cohorts of snails from each of the three strains of snails (Dutch, Belly and Jackson) in each treatment by analyzing the rate of oxygen decrease over time in a closed system (see Methods).
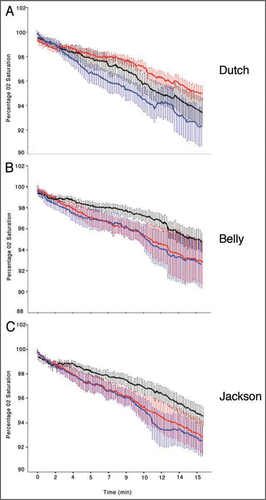
In order to compare each population in each treatment we first needed to determine if the mean rate of O2 consumption was equal across all three populations in PW. We found that there was no significant difference in the rate of oxygen consumption between the three populations (N = 27, p = 0.768, compare PW traces in each population ). We then found that Dutch snails demonstrated significant differences in the rate of oxygen consumption in both CE and SE (). That is, the rate of O2 consumption in CE was significantly less than it was in either PW or SE (, top red trace). Interestingly we found that O2 consumption in SE was also significantly lower than in PW (, middle black trace). When we analyzed the Belly snails O2 consumption we found that there was a significant decrease in the rate on O2 decline in only the SE treatment (, top black trace). That is, the rate of O2 consumption between PW and CE was not significantly different ( bottom overlapping blue and red traces). Lastly, we found that the Jackson snails had a similar response to the three treatments as the Belly snails in that they consumed O2 at the same rate in PW and CE but reduced their O2 consumption when in SE ( bottom overlapping blue and red traces and top black trace respectively). This suggests to us that all three populations of Lymnaea utilize compensatory mechanisms described byCitation10 during exposure to a hypoxic challenge which are suppressed by the detection of a predator. This physiological response is only initiated once a predator is detected.
Discussion
We found previously that Lymnaea respond to the presence of a sympatric predator by significantly altering a number of anti-predator behaviors.Citation3,Citation11,Citation12 In those previous studies lab-reared snails were used exclusively indicating that predator-detection was instinctual and had ‘survived’ lab-rearing for over 250 generations. In a more recent studyCitation1 we found that in Wild Lymnaea exposure to the scent of a sympatric predator but not an allopatric predator elicited anti-predator behaviors including significant changes in aerial respiratory behavior and enhanced long-term memory (LTM) formation. Here we examined the same 3 geographically distinct strains of Lymnaea to determine whether cutaneous respiration was altered as a result of sympatric predator detection.
Crayfish are not sympatric predators of either the Belly or Jackson snails as crayfish are not present in these waters.Citation1 We found that cutaneous respiration was unaltered by CE. However, cutaneous respiration was significantly altered by SE. In both of these Alberta strains cutaneous respiration was immediately and significantly decreased. The situation was somewhat more complicated in the Dutch snails. These snails immediately and significantly decreased their cutaneous respiration in CE. However, these snails also decreased cutaneous respiration in SE, but to a lesser extent than CE. It is important to note, however, that Dutch snails are preyed on by salamanders [e.g., Lissotriton (Triturus) vulgaris the smooth or common newt] so that it was not too surprising that SE elicited some response in these snails. There are no sympatric crustacean predators of any sort in the Alberta watersheds where we collect wild Lymnaea from.
Pulmonate snails use different antipredator responses depending on predator identity.Citation13,Citation14 Some predators (e.g., Crayfish, tench) are localized most often to the pond bottom whilst others (e.g., salamanders) localize themselves to the surface. Thus, snails should, if they wish to avoid the predator (the best anti-predator response), move to the place not frequented by the predator. To effectively do so, snails not only have to detect the predator but have to make the proper decision as to where to ‘hide’. Previous reports demonstrated that when Lymnaea are exposed to crayfish, they crawl to the surface and sometimes even crawl out of the water.Citation15–Citation18 Consistent with those reports are our data showing that Dutch snails in CE increase their aerial respiratory behavior in response to crayfish detection. These data are similar to our previous findings in the Dutchderived laboratory reared snails.Citation3 On the other hand aerial respiration in Dutch snails was not altered in salamander effluent (SE). There are several examples in the literature demonstrating that Lymnaea respond to several predators.Citation13,Citation19 However, this is the first example we know of where this form of differential response has been demonstrated.
Together, the data from all three populations of snails show that predator-detection causes an immediate and significant decrease in cutaneous respiration. When the snails are exposed to a detectable predator scent and are unable to access the surface, snails from all populations decreased their cutaneous respiration. This data is also consistent with our previous findings showing that snails in PW on average consume O2 at a faster rate via cutaneous respiration than they do in predator effluent.Citation3 Therefore, it appears that predator detection alters the so-called ‘compensatory’ response of an organism when faced with a potential hypoxic challenge. As has been described in other publicationsCitation10,Citation20 organisms when sensing a decrease in PO2 initially increase behaviors (the ‘compensatory response’) such as heart rate and respiratory rate to counteract the fall in PO2. Snails that sense the predator did not do this and therefore we expect that these adaptive responses may represent an attempt to minimize O2 requirements, and there-by lessen or avoid hypoxic impairment or damage.Citation21
Methods
Snails.
Lymnaea stagnalis is a cosmopolitan species found worldwide. Here we used three geographically distinct populations of freshly collected ‘wild’ snails from: (1) Polders near Utrecht in The Netherlands (i.e., wild Dutch; latitude: 52° 16′N; longitude: 5° 17E′ and ‘elevation’: −1 m), (2) Six seasonally isolated ponds in the Belly River drainage in Southern Alberta, Canada (i.e., Belly; latitude: 49° 31′N; longitude: 113° 16′W and elevation: 961 m) and (3) A 20-year old humanmade dugout pond (i.e., Jackson snails; latitude: 50° 44′N; longitude: 114° 23′W and elevation: 1,254 m).
Wild Lymnaea stagnalis were identified usingCitation4,Citation5 as well as descriptions from other published works in a similar localities in both The Netherlands and Alberta.Citation6–Citation9 To demonstrate that the Albertan and Dutch snails we collected were the same species, cross breeding experiments were conducted. We found that the progeny of the initial crosses (F1s; e.g., Wild Dutch with Belly) produced viable offspring (F2s). Thus, we concluded that these were in fact the same species.
Snails were collected from ponds in Alberta and polders in The Netherlands in Spring and Summer of 2006–2008, and were then maintained in our laboratory in Calgary before use in the experiments described below.
O2 consumption.
Oxygen consumption (via cutaneous respiration) was measured for snails in pond water (PW,) crayfish effluent (CE) or salamander effluent (SE) in random order in a closed system. Individual snails were placed in a 50 ml Erlenmeyer flask filled with PW, CE or SE and all the air was flushed out when the rubber stopper was placed over the opening of the flask. An oxygen electrode was placed through the stopper and recordings were taken every 10 seconds for 20 minutes. Snails were rested for 48 hours in eumoxic PW and then retested in the next treatment. Snails in all three treatments were compared to “blank” controls where no snail was placed in the chamber to account for electrode O2 consumption. Trials were stopped after 18–20 minutes to ensure that the level of oxygen in the chamber did not decrease beyond 90% saturation. O2 measurements were analyzed using OxyView—PST3-V5.32 02/2004 by PreSens. Experiments were done at room temperature (22°C).
Statistics.
For oxygen consumption experiments, we first compared O2 consumption rate means and slopes measured in PW from each population using ANOVA and multiple linear regression to ensure that the rate of O2 consumption for each populations was similar and therefore comparable. Then we paired averaged time-matched O2 saturation values for each snail in water treatment and again analyzed with ANOVA and multiple linear regression. All statistics were performed on SPSS version 11.0.4 for Macintosh.
Figures and Tables
Figure 1 Non-aerial oxygen consumption in a closed chamber between three populations of Lymnaea stagnalis in PW, CE and SE. (A) O2 consumption of Dutch snails in three water treatments (N = 9, p < 0.01 for each pair-wise comparison). All three water treatments are significantly different from one another. The rate of O2 decrease in Dutch snails is lowest in CE (top red trace) then SE (middle black trace) and is highest in PW (bottom blue trace). (B) O2 consumption on Belly snails was significantly reduced in SE (N = 9, p < 0.01 for each pair-wise comparison, top black trace) but were not significantly different between PW and CE (N = 9, p > 0.05, bottom red and blue overlapping traces). (C) O2 consumption in Jackson snails is similar to that of Belly snails in that SE was significantly reduced compared to PW and CE (N = 9, p < 0.01 for each pair-wise comparison, top black trace) but were not significantly different between PW and CE (N = 9, p > 0.05, bottom red and blue overlapping traces).
Addendum to:
References
- Orr MV, Hittel K, Lukowiak K. Different strokes for different folks: Geographically isolated strains of Lymnaea stagnalis only respond to sympatric predators and have different memory forming capabilities. J Exp Biol 2009; 212:2237 - 2247
- Lukowiak K, Ringseis E, Spencer G, Wildering W, Syed N. Operant conditioning of aerial respiratory behaviour in Lymnaea stagnalis. J Exp Biol 1996; 199:683 - 691
- Orr MV, El-Bekai M, Lui M, Watson K, Lukowiak K. Predator detection in Lymnaea stagnalis. J Exp Biol 2007; 210:4150 - 4158
- Clarke AH. The Freshwater Molluscs of Canada 1981; Ottawa Museum of Natural History,
- Clifford HF. Aquatic Invertebrates of Alberta 1991; Edmonton University of Alberta Press,
- Mooijvog Jw, Jager J, Vanderst Wj. Effects of density levels, and changes in density levels on reproduction, feeding and growth in pond snail Lymnaea stagnalis (L). Proc Koninklijke Nederlandse Akad Van Wetenschappen Ser C Biol Med Sci 1973; 76:245 - 256
- Boag D, Pearlstone P. Life-Cycle of Lymnaea stagnalis (Pulmonata, Gastropoda) in Southwestern Alberta. Can J Zool 1979; 57:353 - 362
- Jager J, Middelburgfrielink N, Mooijvogelaar J, Vandersteen W. Effects of oxygen and food location on behavior in the freshwater snail Lymnaea stagnalis (L). Proc Koninklijke Nederlandse Akad Van Wetenschappen Ser C Biol Med Sci 1979; 82:177 - 180
- Boag D, Thomson C, Vanes J. Vertical-Distribution of young pond snails (Basommatophora, Pulmonata)—implications for survival. Can J Zool 1984; 62:1485 - 1490
- Taylor B, Harris M, Smyth K, Burk M, Lukowiak K, Remmers J. Nitric oxide mediates metabolism as well as respiratory and cardiac responses to hypoxia in the snail Lymnaea stagnalis. J Exp Zool 2003; 295:37 - 46
- Orr MV, Hittel K, Lukowiak K. Comparing memory-forming capabilities between laboratory-reared and wild Lymnaea: learning in the wild, a heritable component of snail memory. J Exp Biol 2008; 211:2807 - 2816
- Lukowiak K, Martens K, Rosenegger D, Browning K, de Caigny P, Orr M. The perception of stress alters adaptive behaviours in Lymnaea stagnalis. J Exp Biol 2008; 211:1747 - 1756
- Turner A, Fetterolf S, Brent R. Predator identity and consumer behavior: differential effects of fish and crayfish on the habitat use of a freshwater snail. Ecology 1999; 118:242 - 247
- Dalesman S, Rundle S, Coleman R, Cotton P. Cue association and antipredator behaviour in a pulmonate snail, Lymnaea stagnalis. Animal Behaviour 2006; 71:789 - 797
- Alexander JE, Covich A. Predation risk and avoidance-behavior in 2 fresh-water snails. Biol Bull 1991; 180:387 - 393
- Covich A, Crowl T, Alexander J, Vaughn C. Predatoravoidance responses in fresh-water decapod-gastropod interactions mediated by chemical stimuli. J North Am Benthol Soc 1994; 13:283 - 290
- Chivers DP, Smith R. Chemical alarm signaling in aquatic predator-prey systems: A review and prospectus. Ecoscience 1998; 5:338 - 352
- McCarthy T, Fisher W. Multiple predator-avoidance behaviours of the freshwater snail Physella heterostropha pomila: responses vary with risk. Freshwater Biol 2000; 44:387 - 397
- Turner A, Montgomery S. Spatial and temporal scales of predator avoidance: experiments with fish and snails. Ecology 2003; 84:616 - 622
- Taylor B, Smyth K, Remmers J, Lukowiak K. Metabolic consequences of hypoxic conditioning in Lymnaea stagnalis. Adv Exp Med Biol 2001; 499:225 - 229
- Hochachka P, Buck L, Doll C, Land S. Unifying theory of hypoxia tolerance: molecular/metabolic defense and rescue mechanisms for surviving oxygen lack. Proc Natl Acad Sci USA 1996; 93:9493 - 9498