Abstract
For evolution and maintenance of the social systems of insect colonies, caste production should be controlled in response to external cues so that caste ratio in the colony is kept in an optimal range. Recent developments using artificial diet rearing techniques have revealed an underlying mechanism for adaptive control of caste production in a social aphid, Tuberaphis styraci, which has a sterile soldier caste in the 2nd instar. Aphid density was the proximate cue that acts on 1st instar nymphs and embryos to induce soldier differentiation. The final determination of soldier differentiation occurred postnatally, probably at a late 1st instar stage. Direct contact stimuli from live non-soldier aphids mediated the density effect. While coexisting non-soldiers facilitated soldier differentiation in 1st instar nymphs, coexisting soldiers acted to suppress such differentiation. These results suggest that caste production in aphid colonies is controlled by positive and negative feedback mechanisms consisting of density-dependent induction and suppression of soldier differentiation. Here, we demonstrate the mechanisms that coordinate aphid society, and provide a striking case of clonal superorganism system where simple responses of colony members to local extrinsic stimuli are integrated into a highly organized regulation of the whole colony.
Introduction
Organisms have evolved through major transitions in the long history of life. Evolution of sociality is one of the important transitions and has brought ecological success to organisms. A colony of social insects, with hundreds, thousands or millions of individuals integrated into a cohesive and homeostatic system with division of labor, is one of the most impressive biological entities in nature. It is of great interest to understand the mechanisms underlying the regulation and control of the biological system referred to as superorganism.Citation1
In colonies of highly social insects, some individuals are engaged in reproduction, whereas others produce few or no offspring and comprise specialized castes that are characterized by distinct behavioral and morphological traits for altruistic functions. Morphologically and reproductively differentiated castes are well known in social groups like bees, ants and termites,Citation2 but such castes have also evolved in less-studied groups like aphids.Citation3
Some aphids produce individuals that altruistically sacrifice their own reproduction for the benefit of their colony mates. Such individuals are called “soldiers” because their primary social role is usually defense, although some soldiers also play non-defensive altruistic roles such as housekeepingCitation4,Citation5 and gall repair.Citation6–Citation8 In highly social aphids, soldiers are morphologically differentiated from the normal nymphs and are unable to grow, constituting a sterile caste.Citation9–Citation12 So far, about 60 soldier-producing aphids have been found from two aphid subfamilies, the Pemphiginae and the Hormaphidinae, where morphologically and reproductively specialized soldier castes have evolved at least four times independently.Citation9–Citation13
Giving up their personal reproductive potential, these altruistic castes ensure transmission of their gene copies only by contributing to colony success. Production of the sterile castes improves colony success through their altruistic tasks such as defense and housekeeping, at the expense of intrinsic growth rate of the colony. On account of the trade-off between reproduction and altruistic functions, there should be an optimal investment to altruistic castes in a particular environment.Citation11,Citation14–Citation16 Since environmental factors such as predation, competition and available resources fluctuate, the optimal caste ratio is predicted to shift with time in an unpredictable manner. Therefore, mechanisms of controlling the caste investment in response to external cues, whereby the caste ratio in the colony is controlled within an optimal range, are important for evolution and maintenance of the social system.
However, the processes underlying the adaptive and flexible control of caste differentiation in social insect colonies are not well understood. Experimental evidence for adaptive shifts in caste ratio is limited,Citation17–Citation19 probably because manipulation of environmental factors that may influence caste ratio is usually difficult in complex and homeostatic insect social systems. In particular, social aphids, most of which live on tall trees or bamboos, are thought to be difficult to analyze in the laboratory.
Development of an Artificial Diet Rearing System and Experimental Model of Social Aphids
Tuberaphis styraci is representative of highly social aphids and forms large coral-shaped galls on the tree Styrax obassia (). In the galls, adult females parthenogenetically produce monomorphic 1st instar nymphs. When they molt into 2nd instar, two distinct morphs, normal 2nd instar nymphs and soldiers, are produced (). Normal 2nd instar nymphs grow to adult and reproduce, whereas soldiers neither grow nor reproduce but are specialized for altruistic tasks, e.g., colony defense and housekeeping ().Citation20,Citation21
Recently, we developed an artificial diet rearing technique for T. styraci.Citation22 On a small petri dish filled with synthetic liquid diet, we were able to maintain T. styraci in the laboratory for longer than two months through three successive generations. On the artificial diet, the aphids survived, grew and reproduced as they were in native galls. Moreover, soldiers were produced on the diet, and they exhibited attacking and cleaning behaviors even under the extremely artificial but experimentally manageable condition. These findings prompted us to perform experimental approaches to various biological aspects of the social aphid. Here, we review our recent studies on the mechanism underlying adaptive control of soldier caste differentiation in T. styraci using the artificial diet technique.
Density-Dependent Soldier Production in T. styraci
In social aphids, a number of factors have been shown or suggested to affect the proportion of soldiers in a colony of social aphids: gall size,Citation23 colony size,Citation24,Citation25 proportion of unwinged adults,Citation25 clonal mixing,Citation26 predator abundance,Citation27 ant attendance, Citation19,Citation28 host plant conditionCitation29 and seasonal change.Citation25,Citation30–Citation32 To date, however, direct environmental cues that control aphid soldier production have been unequivocally identified.
We surveyed population dynamics and colony composition in natural galls of T. styraci to statistically identify environmental factors related to soldier production.Citation33 Regression analyses indicated that soldier proportion is significantly correlated with colony size (r = 0.586, p < 0.05) and aphid density (r = 0.857, p < 0.001). In stepwise regression analyses, aphid density exhibited a highly significant correlation (r = 0.857, p < 0.0001) with soldier proportion. These results suggested that crowding is a candidate factor that controls soldier production in T. styraci.
Using an artificial diet rearing system, we investigated the effect of crowding in the laboratory.Citation33 When a single adult was reared in a chamber, few soldiers were produced. By contrast, when 10 to 20 adults were maintained in a chamber, many soldiers were produced. After a month of maintenance, soldier proportion in the offspring reached around 50% under the crowded conditions. These results indicated that crowded condition induces soldier production in T. styraci.
Then, we designed a series of artificial diet experiments by manipulating aphid density or colony size. The results revealed that soldiers are produced in a density-dependent manner rather than in a colony size-dependent manner. Thus, it was concluded that high density induces soldier production in T. styraci, which is in perfect agreement with the data from field-collected galls.Citation33
Proximate Mechanisms for Density-Dependent Soldier Differentiation
In T. styraci, female adults parthenogenetically produce monomorphic 1st instar nymphs, and the nymphs molt into normal 2nd instar nymphs and soldiers. Therefore, the nymphs destined to be soldiers experienced the density treatment through two developmental phases: as embryos in maternal body and as 1st instar nymphs. To identify the developmental stage responsive to high density, we performed combinatorial prenatal and postnatal density treatments.Citation34 In our experiments, either prenatal high density or postnatal high density could induce soldier differentiation of the treated aphids, indicating that both newborn nymphs and embryos in maternal bodies are responsive to high aphid density.
Interestingly, 1st instar nymphs of T. styraci were able to become either normal 2nd instar nymphs or soldiers in response to density.Citation34 Therefore, the final determination of soldier differentiation must occur postnatally, probably at a late 1st instar stage. The bipotency of 1st instar nymphs might be important for the aphid colonies to realize a flexible control of soldier production in response to a fluctuating environment.
In our experiments, prenatal high density alone induced around 10% soldiers, and postnatal high density alone also induced about 10% soldiers. However, the combination of prenatal and postnatal high densities resulted in not 20% but 40% soldiers.Citation34 These results indicated that a combination of prenatal density and postnatal density enhances soldier differentiation in a synergistic manner. Continuous exposure to high density is important for the effective induction of soldiers in T. styraci.
Under high density conditions, insects must experience a number of environmental changes such as frequent contact with other individuals, increased amount of wastes, elevated concentration of pheromones and other chemicals. To identify the causal agent of the density-dependent soldier differentiation, we conducted artificial diet experiments in which adult insects of T. styraci were exposed to normal nymphs, soldiers, dead normal nymphs, shed skins, honeydew globules and excreted wax.Citation35 Among the factors examined, only live normal nymphs effectively induced soldier production. These results indicated that coexistence with live normal insects is important for the density-dependent induction of soldiers.
Then, what is the nature of the soldier-inducing cue associated with normal aphids? The level of volatile chemicals like pheromones released by normal aphids may increase under crowded conditions. Salivary components secreted by normal aphids into the diet may be perceived by other feeding aphids. To gain further insights into the nature of the soldier-inducing cue, we examined whether the cue is mediated by volatile/diffusible factors or direct contact.Citation35 The artificial diet experiments using partitioned and non-partitioned chambers rejected involvement of these factors, and suggested that direct contact between live normal aphids is the principal cue that mediates the information of aphid density.
Density-Dependent Control Mechanisms over Induction and Suppression of Soldier Differentiation
In natural and laboratory colonies of T. styraci, higher density of insects induced production of more soldiers.Citation36 However, the relationship was not linear, and the soldier proportion appeared to reach a plateau under high aphid densities. One possible explanation for the plateau is that T. styraci is physiologically constrained to produce a limited proportion of nymphs competent to become soldiers. Another possibility is that soldiers themselves are involved in the regulation of soldier proportion in colonies of T. styraci.
To test the latter possibility, we designed an artificial diet experiment to examine how soldier production is affected by coexisting soldiers and non-soldiers.Citation36 The soldier proportion attained in the presence of 60 normal nymphs was higher than that in the presence of 30 normal nymphs, whereas the soldier proportion attained in the presence of 30 normal nymphs plus 30 soldiers was significantly lower than those in the absence of soldiers. These results indicated that pre-existing soldiers suppress further soldier production, suggesting that soldier proportion in colonies of T. styraci may be controlled by a soldier-mediated negative feedback mechanism.
On the basis of above results, we propose a model for the mechanisms of caste differentiation and control in colonies of T. styraci (). In the life history of T. styraci (Aoki & Kurosu 1989, 1990), the positive and negative feedback mechanisms might enable an adaptive reproductive allocation. In a young gall with only a small number of insects, few soldiers are induced because of low aphid density, which maximizes the intrinsic growth rate of the colony and leads to prompt attainment of a large colony size when the nutritional condition of the gall is good. As the colony size increases, aphid density in the gall is quickly elevated, available resources and space for gall inhabitants are limited, and increasing aphid biomass in the gall becomes attractive for predators. At this stage, a large number of soldiers are produced in response to the high aphid density, which suppresses the colony growth, defends the colony from predators, and therefore meets the ecological requirements for the colony. Furthermore, even when the colony size becomes very large in maturity, investment in soldier production is controlled within an appropriate range by the negative feedback mechanism. This scenario is, however, at present hypothetical and to be verified by further studies on the ecological aspects of T. styraci.
In both natural and laboratory colonies, the soldier proportion consistently reached a plateau at around 50% under high aphid densities.Citation36 The soldier proportion may be realized through a dynamic equilibrium between induction by non-soldier aphids and suppression by soldiers as proposed. Alternatively, T. styraci may be physiologically constrained to be able to produce a limited proportion of nymphs competent to become soldiers. The flexibility and constraint underlying the soldier differentiation are important for understanding the aphid social system and to be investigated further.
Why maximal soldier proportion is around 50% is intriguing. It is conceivable that the value reflects the optimal soldier investment in mature galls of T. styraci. To quantitatively evaluate the optimal soldier investment, the cost and benefit of soldier production should be examined in the ecology and life cycle of T. styraci. It should be also taken into account that soldiers of T. styraci perform colony defense as well as housekeeping. While predatory attacks are rather episodic and unpredictable, colony wastes are constantly produced in the galls. The relative importance of these social tasks for survival and reproductive success of the aphid colonies is of great interest but are totally unknown.
Physiological Mechanisms of Soldier Differentiation
In ants, bees, wasps and termites, not only volatile pheromones but also surface molecules such as cuticle hydrocarbons play important roles in their social communications and interactions. Citation37–Citation39 Our results indicated that information of aphid density was transmitted between live aphid individuals by direct contact. Citation35 In addition, soldier production was induced by coexisting normal aphids, and was suppressed by coexisting soldiers.Citation36 Probably, reproductive aphids recognize some difference between normal aphids and soldiers, and differentially respond to them. It will be of interest to compare the composition of surface non-volatiles between normal aphids and soldiers of T. styraci. Contact chemicals such as cuticle hydrocarbons might be involved in the caste differentiation and control in the aphid social system.
In our study, not only postnatal density treatment but also prenatal density treatment induced soldier differentiation in T. styraci,Citation34 indicating that information of density perceived by the mother aphid is somehow transmitted to embryos inside the body. To explain this process, involvement of endocrine system is suspected. In ants, bees and termites, juvenile hormone has been shown to regulate caste and task differentiation.Citation40–Citation45 Treatments of T. styraci with analogs and antagonists of juvenile hormone and other insect hormones would lead to understanding of physiological mechanisms underlying the caste differentiation.
Soldier Caste as an Expression of Aphid Polyphenism
Aphids are known for their complex life cycle and extreme polyphenism. Citation46 In many aphid species, crowded conditions induce differentiation into winged morphs,Citation47 which is reminiscent of the soldier differentiation in T. styraci. Some common mechanisms might underlie the differentiation of these and other aphid morphs. Photoperiod, nutritional conditions, temperature and other factors also affect the differentiation of winged aphids.Citation47 In addition to density, these factors may influence the soldier differentiation in T. styraci. In our preliminary experiments, density-dependent regulation of wing polyphenism was observed in T. styraci under a short day condition (8L:16D). Notably, proportion of alates was not directly correlated with proportion of soldiers but negatively correlated with proportion of unwinged normal aphids in the colonies (Shibao H, in preparation). These data suggest that the differentiation between alates and soldiers occurs at an early nymphal or embryonic stage, and that T. styraci is physiologically constrained to produce a limited proportion of soldier-destined nymphs. Further studies are needed to examine these possibilities.
Perspective
Despite technical difficulties with the non-model insects, recent development in molecular genetics and genomics has unveiled some intriguing molecular aspects relevant to social traits in bees, ants, termites and aphids.Citation48–Citation58 T. styraci provides a useful model system to understand various aspects of the aphid sociality. Since soldiers and normal nymphs are parthenogenetically produced by the same mother, the phenotypic differences between them must be due to the differential gene expression between them. The soldier induction technique using density treatment will enable us to identify the specific genes expressed in the process of soldier differentiation. Development of artificial diet rearing system for other social aphids such as Pseudoregma bambucicola and Colophina arma, those which possess a 1st instar soldier caste of independent evolutionary origin, will allow us to conduct comparative studies on aphid social mechanisms in further depth,Citation59,Citation60 which should provide insights into such intriguing aspects of the aphid social biology.
Figures and Tables
Figure 1 Ecology and life cycle of Tuberaphis styraci. (A) A mature 2nd-year-gall. (B) Two types of 2nd instar nymphs. Left, soldier. Right, normal nymph. Soldiers are easily recognized by sclerotized cuticle and greenish color, in contrast to soft cuticle and yellowish color of normal 2nd instar nymphs. Bar = 1 mm. (C) Soldiers attacking a lacewing larva. (D) Soldiers pushing honeydew globules with their heads. Here we summarize the ecology and life cycle of T. styraci. This aphid has a biennial life cycle on the host tree Styrax obassia.Citation20,Citation21 A fundatrix that hatched from an overwintered egg forms a small gall, and next year the incipient gall grows into a large gall, around 10 cm in diameter and coral-shaped. In the gall, adult females parthenogenetically produce monomorphic 1st instar nymphs. When they molt into 2nd instar, two distinct morphs, normal 2nd instar nymphs and soldiers, appear. Normal 2nd instar nymphs grow to adult and reproduce, whereas soldiers neither grow nor reproduce but are specialized for altruistic tasks, colony defense and housekeeping. Soldiers are gathering around small holes on the underside of the gall, guarding the openings, and cleaning the gall by pushing honeydew globules, shed skins and corpses out of the holes. Encountering intruders, soldiers aggressively attack them by stinging with their stylets and injecting toxic saliva. It was recently shown that the soldiers produce a venomous protease in their gut and inject it into predators for defense.Citation57,Citation58 Aphid predators such as syrphid maggots and lacewing larvae are tormented, paralyzed or killed by the attack, and usually drop off the gall surface with attacking soldiers attached to them. Therefore, the attacking behavior of soldiers is highly self-sacrificing. Mature large galls of T. styraci sometimes contain over 20,000 insects, more than a half of which may be soldier individuals. In late August to mid October, alate sexuparae, which migrate to another S. obassia trees and produce the sexual generation, emerge from the mature gall via the exit holes. Males and females produced by the sexuparae copulate and produce eggs, which hatch in the next year.
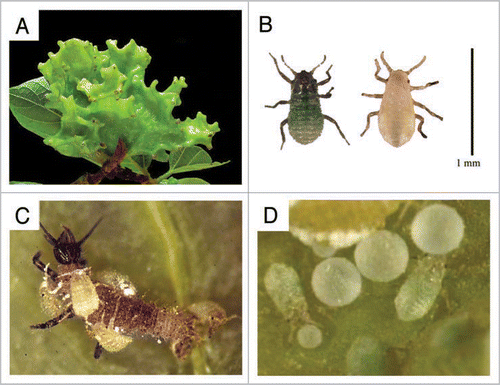
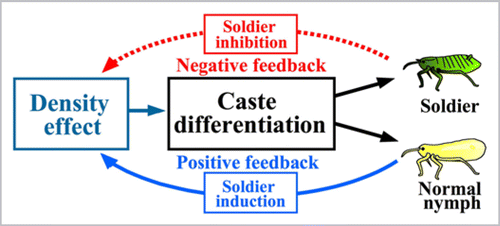
Figure 2 A model for the mechanism of caste differentiation and control in colonies of T. styraci. At the individual level, two factors are involved in soldier differentiation: normal aphids as an inducing cue and soldiers as a suppressing cue. At the colony level, positive and negative feedback is operating to control soldier production: higher density of normal aphids induces soldier production, whereas successive elevation of soldier proportion suppresses further soldier production, whereby investment to soldier production at the colony level is controlled within an appropriate range. In this way, simple responses of aphid individuals to local environmental cues can be integrated into a highly organized regulation of the whole colony.
Acknowledgements
This research was supported by a grant from the Kazato Research Foundation, a Grant-in-Aid (no. 18770012) for Scientific Research from the Japan Society for the Promotion of Science, and a 21st COE (Centers of Excellence) Program of the Research Center of Integrated Sciences at the University of Tokyo financed by the Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
- Seeley TD. The Social Physiology of Honey Bee Colonies 1995; Cambridge, MA Harvard Univ Press
- Wilson EO. The Insect Societies 1971; Cambridge, MA Belknap, Harvard Univ Press
- Aoki S. Colophina clematis (Homoptera: Pemphigidae), an aphid species with “soldiers”. Kontyu 1977; 45:276 - 282
- Aoki S. Occurrence of a simple labor in a gall aphid Pemphigus dorocola (Homoptera, Pemphigidae). Kontyu 1980; 48:71 - 73
- Benton TG, Foster WA. Altruistic housekeeping in a social aphid. Proc R Soc Lond B 1992; 247:199 - 202
- Kurosu U, Aoki S, Fukatsu T. Self-sacrificing gall repair by aphid nymphs. Proc R Soc Lond B 2003; 270:12 - 14
- Pike N, Foster W. Fortress repair in the social aphid species Pemphigus spyrothecae. Anim Behav 2004; 67:909 - 914
- Kutsukake M, Shibao H, Uematsu K, Fukatsu T. Scab formation and wound healing of plant tissue by soldier. Proc R Soc Lond B 2009; 276:1555 - 1563
- Aoki S. Ito Y, Brown JL, Kikkawa J. Evolution of sterile soldiers in aphids. Animal Societies: Theories & Facts 1987; Tokyo Japan Scientific Societies Press, 53 - 65
- Ito Y. The evolutionary biology of sterile soldiers in aphids. Trends Ecol Evol 1989; 4:69 - 73
- Stern DL, Foster WA. The evolution of soldiers in aphids. Biol Rev 1996; 71:27 - 79
- Pike N, Foster WA. Korb J, Heinze J. The ecology of altruism in a clonal insect. Ecology of Social Evolution 2008; Berlin Springer, 37 - 56
- Fukatsu T, Sarjiya A, Shibao H. Soldier caste with morphological and reproductive division in the aphid tribe Nipponaphidini. Insectes Soc 2005; 52:132 - 138
- Oster GF, Wilson EO. Caste and Ecology in the Social Insects 1978; Princeton Princeton Univ Press
- Akimoto S. Ecological factors promoting the evolution of colony defense in aphids: computer simulations. Insectes Soc 1996; 43:1 - 15
- Hasegawa E. The optimal caste ratio in polymorphic ants: estimation and empirical evidence. Am Nat 1997; 149:706 - 722
- Passera L, Roncin E, Kaufmann B, Keller L. Increased soldier production in ant colonies exposed to intraspecific competition. Nature 1996; 379:630 - 631
- Harvey AJ, Corley LS, Strand MR. Competition induces adaptive shifts in caste ratios of a polyembryonic wasp. Nature 2000; 406:183 - 186
- Shingleton AW, Foster WA. Ant attendance influences soldier production in a social aphid. Proc R Soc Lond B 2000; 267:1863 - 1868
- Aoki S, Kurosu U. Soldiers of Astegopteryx styraci (Homoptera, Aphidoidea) clean their gall. Jpn J Entomol 1989; 57:407 - 416
- Aoki S, Kurosu U. Biennial galls of the aphid Astegopteryx styraci on a temperate deciduous tree, Styrax obassia. Acta Phytopathol Entomol Hungarica 1990; 25:57 - 65
- Shibao H, Kutsukake M, Lee JM, Fukatsu T. Maintenance of soldier-producing aphids on an artificial diet. J Insect Physiol 2002; 48:495 - 505
- Stern DL, Aoki S, Kurosu U. A test of geometric hypotheses for soldier investment patterns in the gall producing tropical aphid Cerataphis fransseni (Homoptera: Hormaphididae). Insectes Soc 1994; 41:457 - 460
- Shibao H. Reproductive schedule and factors affecting soldier production in the eusocial bamboo aphid Pseudoregma bambucicola (Homoptera, Aphididae). Insectes Soc 1999; 46:378 - 386
- Ijichi N, Shibao H, Miura T, Matsumoto T, Fukatsu T. Analysis of natural colonies of a social aphid Colophina arma: population dynamics, reproductive schedule, and survey for ecological correlates with soldier production. App Entomol Zool 2005; 40:239 - 245
- Abbot P, Withgott JH, Moran NA. Genetic conflict and conditional altruism in social aphid colonies. Proc Natl Acad Sci USA 2001; 98:12068 - 12071
- Shibao H. Social structure and the defensive role of soldiers in a eusocial bamboo aphid, Pseudoregma bambucicola (Homoptera: Aphididae): a test of the defence-optimization hypothesis. Res Popul Ecol 1998; 40:325 - 333
- Shibao H, Morimoto M, Okumura Y, Shimada M. Fitness costs and benefits of ant attendance and soldier production for the social aphid Pseudoregma bambucicola (Homoptera: Aphididae: Hormaphidinae). Sociobiology 2009; 54:1 - 26
- Sakata K, Ito Y, Yukawa J, Yamane Sk. Ratio of sterile soldiers in the bamboo aphid, Pseudoregma bambucicola (Homoptera: Pemphigidae), colonies in relation to social and habitat conditions. Appl Entomol Zool 1991; 26:463 - 468
- Akimoto S. Shift in life-history strategy from reproduction to defense with colony age in the galling aphid Hemipodaphis persimilis producing defensive first-instar larvae. Res Popul Ecol 1992; 34:359 - 372
- Sunose T, Yamane Sk, Tsuda K, Takasu K. What do the soldiers of Pseudoregma bambucicola defend?. Jpn J Entomol 1991; 59:141 - 148
- Tanaka S, Ito Y. Reversal of caste production schedule in an eusocial aphid, Pseudoregma koshunensis. Naturwissenschaften 1994; 81:411 - 413
- Shibao H, Kutsukake M, Fukatsu T. Density triggers soldier production in a social aphid. Proc R Soc Lond B 2004; 271:71 - 74
- Shibao H, Lee JM, Kutsukake M, Fukatsu T. Aphid soldier differentiation: density acts on both embryos and newborn nymphs. Naturwissenschaften 2003; 90:501 - 504
- Shibao H, Kutsukake M, Fukatsu T. Contact with non-soldiers acts as a proximate cue of densitydependent soldier production in a social aphid. J Insect Physiol 2004; 50:143 - 147
- Shibao H, Kutsukake M, Fukatsu T. Densitydependent induction and suppression of soldier differentiation in an aphid social system. J Insect Physiol 2004; 50:995 - 1000
- Lefeuve P, Bordereau C. Soldier formation regulated by a primer pheromone from the soldier frontal gland in a higher termite, Nasutitermes lujae. Proc Natl Acad Sci USA 1984; 81:7665 - 7668
- Hölldobler D, Wilson EO. The Ants 1990; Cambridge, MA Belknap, Harvard Univ. Press
- Vander Meer RK, Breed MD, Winston ML, Espelie KE. Pheromone Communication in Social Insects: Ants, Wasps, Bees, and Termites 1998; Boulder Westview Press
- Robinson GE. Regulation of honey-bee age polyethism by juvenile-hormone. Behav Ecol Sociobiol 1987; 20:329 - 338
- Robinson GE. Regulation of division of labor in insect societies. Ann Rev Entomol 1992; 37:637 - 665
- Luscher M. Luscher M. Evidence for an endocrine control of determination in higher termites. Phase and Caste Determination in Insects 1976; Oxford Pergamon 91 - 103
- Nijhout HF, Wheeler DE. Juvenile hormone and the physiological basis of insect polymorphisms. Quart Rev Biol 1982; 57:109 - 133
- Nijhout HF. Insect Hormones 1994; Princeton Princeton Univ. Press
- Wheeler DE. Developmental and physiological determinants of caste in social Hymenoptera: evolutionary implications. Am Nat 1986; 128:13 - 34
- Miyazaki M. Minks AK, Harrewijn P. Polymorphism and morph determination. Aphids: Their Biology, Natural Enemies and Control 1987; 2a:Amsterdam Elsevier 27 - 50
- Kawada K. Minks AK, Harrewijn P. Polymorphism and morph determination. Aphids: Their Biology, Natural Enemies and Control 1987; 2a:Amsterdam Elsevier 255 - 266
- Abouheif E, Wray GA. Evolution of the gene network underlying wing polyphenism in ants. Science 2002; 297:249 - 252
- Ben-Shahar Y, Robichon A, Sokolowski MB, Robinson GE. Influence of gene action across different time scales on behavior. Science 2002; 296:741 - 744
- Krieger MJB, Ross K. Identification of a major gene regulating complex social behavior. Science 2002; 295:328 - 332
- Krieger MJB, Ross K. Molecular evolutionary analyses of the odorant-binding protein gene Gp-9 in fire ants and other Solenopsis species. Mol Biol Evol 2005; 22:2090 - 2103
- Bulmer MS, Crozier RH. Duplication and diversifying selection among termite antifungal peptide. Mol Biol Evol 2004; 21:2256 - 2264
- Robinson GE, Grozinger CM, Whitfield CW. Sociogenomics: social life in molecular terms. Nat Rev Genet 2005; 6:257 - 270
- Drapeau MD, Albert S, Kucharski R, Prusko C, Maleszka R. Evolution of the yellow/major jelly protein family and the emergence of social behavior in honey bees. Genome Res 2006; 16:1385 - 1394
- Foret S, Maleszka R. Function and evolution of a gene family encoding odorant binding-like proteins in a social insect, the honey bee (Apis mellifera). Genome Res 2006; 16:1404 - 1413
- The Honeybee Genome Sequencing Consortium. Insights into social insects from the genome of the honeygee Apis mellifera. Nature 2006; 443:931 - 949
- Kutsukake M, Shibao H, Nikoh N, Morioka M, Tamura T, Hoshino T, et al. Venomous protease of aphid soldier for colony defense. Proc Natl Acad Sci USA 2004; 101:11338 - 11343
- Kutsukake M, Nikoh N, Shibao H, Rispe C, Simon JC, Fukatsu T. Evolution of soldier-specific venomous protease in social aphids. Mol Biol Evol 2008; 25:2627 - 2641
- Ijichi N, Shibao H, Miura T, Matsumoto T, Fukatsu T. Soldier differentiation during embryogenesis of a social aphid Pseudoregma bambucicola. Entomol Sci 2004; 7:141 - 153
- Ijichi N, Shibao H, Miura T, Matsumoto T, Fukatsu T. Comparative analysis of caste differentiation during embryogenesis of social aphids whose soldier castes evolved independently. Insectes Soc 2005; 52:177 - 185